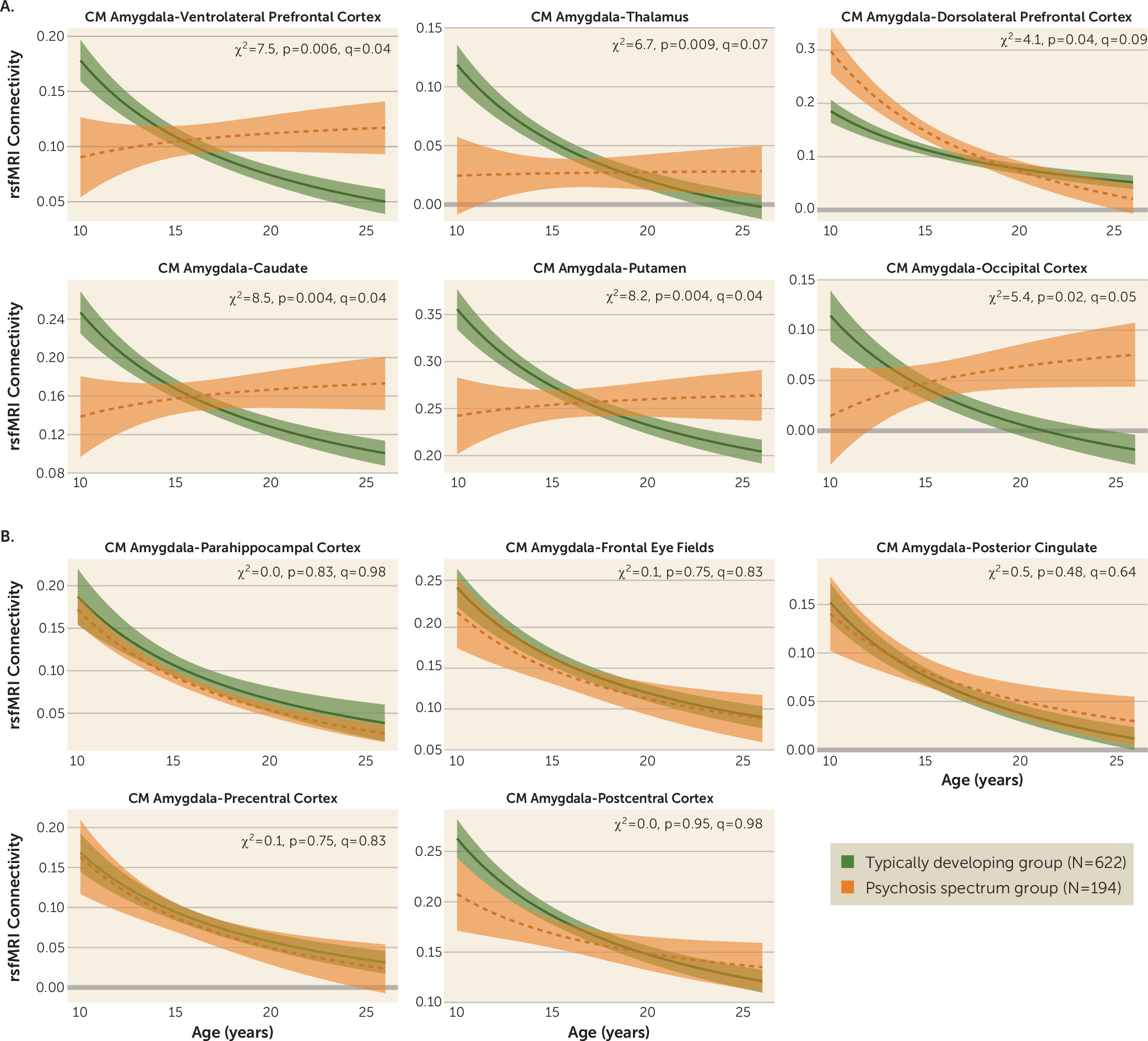
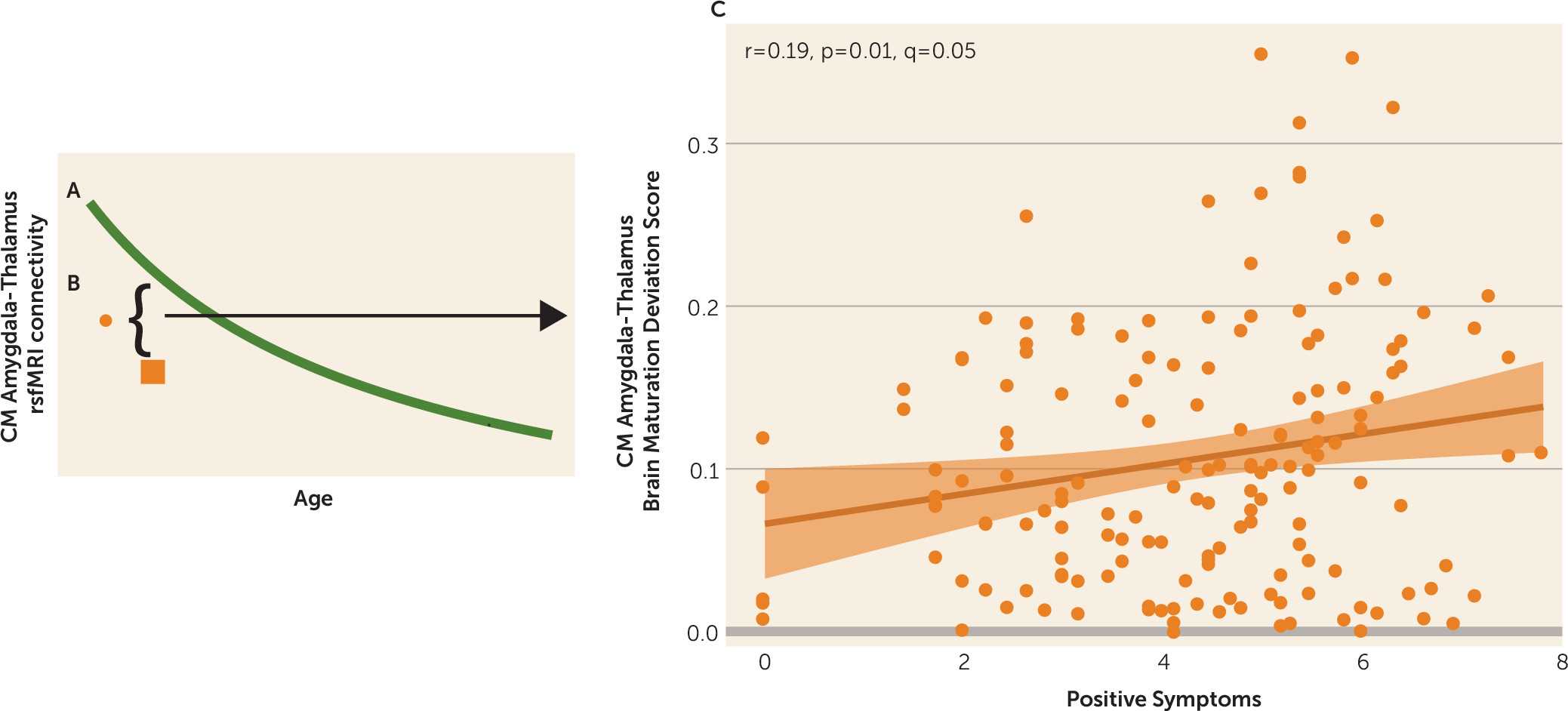
We examined age-associated disruptions in amygdala functional connectivity in youths with psychosis spectrum disorders and explored how alterations in neurodevelopmental connectivity may be related to psychotic symptoms. First, we developed normative amygdala connectivity growth charts in typically developing youths and verified that the strongest age-associated changes occur in connectivity between the centromedial amygdala and multiple brain regions, with decreases in connectivity occurring as age increased, confirming our previous results (
13). Next, we showed that the psychosis spectrum group failed to show typical age-associated decreases in connectivity between the centromedial amygdala and these distinct brain regions: the striatum, thalamus, lateral prefrontal cortex, and occipital cortex. Age-associated alterations in centromedial amygdala–putamen connectivity and centromedial amygdala–occipital cortex connectivity were unique to the psychosis spectrum group; youths in the other psychopathology group did not exhibit these age-associated deviations. Exploratory analyses revealed that greater age-related deviations in centromedial amygdala–thalamus functional maturation were associated with greater positive symptoms in the psychosis spectrum group. Our results provide a novel view of developmental alterations in functional connectivity in psychosis spectrum disorders, implicating alterations during discrete developmental windows within neural circuits implicated in a wide array of cognitive and emotional processes.
Developmental Alterations in Centromedial Amygdala Connectivity in Youths With Psychosis Spectrum Disorders
In line with our previous findings, significant typical developmental functional connectivity decreases occurred between the centromedial amygdala and multiple brain regions (
13). These results are consistent with previous developmental neuroimaging resting-state fMRI studies reporting decreases in subcortical-cortical connectivity into adulthood (
36–
39). In comparison to typically developing youths, those with psychosis spectrum disorders exhibited reduced connectivity between the centromedial amygdala and the ventrolateral prefrontal cortex, striatum, thalamus, and occipital cortex during late childhood and early adolescence, with a lack of normative decreases from adolescence to adulthood. These findings suggest either that there is an accelerated developmental decrease in amygdala connectivity in psychosis preceding the normative timetable or that the earlier age at onset reflects deterioration of this circuitry, as is evident later in adulthood. Normatively, decreases in connectivity can be seen as a period of specialization that occurs at a critical time when higher-level systems are becoming established to form adult trajectories. The lack of a marker of specialization through adolescence could reflect impairments in optimal specialization that could contribute to abnormal processing of executive affective information processing in psychosis.
Many of the regions that exhibited disrupted age-associated amygdala connectivity in the psychosis spectrum group are related to perception and salience (e.g., thalamus, striatum, and occipital cortex [
40–
47]). A primary function of the amygdala is to determine what is salient in one’s environment and to facilitate learning for these items (
48–
50). Projections of the amygdala to the thalamus are thought to modulate attentional orientation and arousal, broadly speaking (
51). Projections of the amygdala to the visual cortex are known to enhance sensitivity, discrimination, and subjective vividness of perceived stimuli (
52). Finally, projections of the amygdala to the striatum are thought to provide an interface between the amygdala and dopamine systems, which are known to regulate motivation, learning, and behavioral activation (
53). Thus, abnormal connectivity of the amygdala with the thalamus, visual cortex, and striatum could reflect processes that result in the misattribution of salience to stimuli in psychosis, as well as a facilitation of learning associations about threat-related items. Critically, this type of aberrant salience processing may provide the foundation for higher-order features of positive symptoms, such as delusions (
54).
Connections between the centromedial amygdala and lateral prefrontal regions also exhibited a disruption in age-associated changes in the psychosis spectrum group. During late childhood and early adolescence, the psychosis spectrum group exhibited reduced amygdala–ventrolateral prefrontal cortex connectivity; however, during adulthood the psychosis spectrum group exhibited increased connectivity between these two regions. Amygdala–ventrolateral prefrontal cortex connectivity is necessary during the reappraisal phase of regulating one’s emotions (
55–
59), and how these two structures interact during emotion regulation changes during adolescent development (
60). Indeed, impairments in lateral prefrontal–mediated circuitry are related to emotional deficits typically observed in psychosis (
61–
63) and age-associated amygdala–ventrolateral prefrontal cortex functional connectivity alterations during an affective labeling task have been observed in youths at clinical high risk for developing psychosis (
64). Thus, centromedial amygdala–ventrolateral prefrontal cortex age-associated disruptions may underlie the emotional dysregulation that often precedes and predicts increased psychotic symptoms (
65–
67). Studies using experience sampling have shown that adults with schizophrenia report more intense negative emotions and greater social stress than healthy control subjects (
68–
70). The heightened amygdala–ventrolateral prefrontal cortex connectivity we observed in adults experiencing psychosis spectrum symptoms may reflect underlying biological vulnerability to psychosis onset, which interacts with these environmental stressors. Studies integrating experience sampling methods (
71) with neuroimaging in youths at high risk for developing psychosis are necessary to test this hypothesis.
We also observed a unique, altered age-associated pattern of centromedial amygdala–dorsolateral prefrontal cortex connectivity in the psychosis spectrum group compared with the typically developing control group. During late childhood and early adolescence, the psychosis spectrum group exhibited increased centromedial amygdala–dorsolateral prefrontal cortex coupling compared with the typically developing group. This group difference was no longer present in adulthood, with both the typically developing and the psychosis spectrum groups exhibiting similar levels of connectivity. Previously, in adults with schizophrenia, absent or reduced amygdala–dorsolateral prefrontal cortex functional connectivity has been observed during emotional distraction during a working memory task (
72) and at rest (
7,
21). Our developmentally sensitive results of increased centromedial amygdala–dorsolateral prefrontal cortex connectivity in the psychosis spectrum group during late childhood and early adolescence contrast with these findings and highlight the importance of examining how developmental stage may affect the directionality and interpretation of brain connectivity (
73). Reduced GABA levels are consistently observed in the prefrontal cortex in schizophrenia. While GABA-ergic deficits have not been identified in the amygdala in schizophrenia, the majority of neurons projecting from the centromedial amygdala are GABA-ergic (
74). Altered connections may generate down-regulation of GABA interneuron activity in the prefrontal cortex, resulting in a lack of inhibition, which may be responsible for the increased connectivity observed between the amygdala and dorsolateral prefrontal cortex during late childhood and early adolescence in youths with psychosis spectrum disorders.
In summary, we identified developmentally sensitive alterations in cortico-limbic and intralimbic resting-state fMRI connectivity in youths with psychosis spectrum disorders. These neurodevelopmental alterations provide support for multiple theories associated with schizophrenia and complement the growing body of literature that shows progressive maturational disturbances in those who go on to develop psychosis (
75–
78).
Limitations
Our study was limited by the fact that cross-sectional data were available only for the psychosis spectrum group. Thus, the neurodevelopmental trajectories in psychosis spectrum disorders do not reflect within-person change. Our cross-sectional sample cannot definitively show whether our results are due to altered development or abnormalities due to time of onset of psychosis. Thus, longitudinal studies of youths with psychosis spectrum disorders, with multiple visits per individual, can extend our understanding of psychosis by identifying how the shape and rate of maturation of subject-specific developmental trajectories in youths with psychosis spectrum disorders diverge, converge, or remain stable in comparison to typical development (
82–
84). Furthermore, many developmental changes that occur during adolescence are nonlinear, and these patterns are most accurately captured with longitudinal analyses (
85). Additionally, psychotic symptoms are dynamic and change over time (
86,
87), and these changes need to be taken into account when characterizing neurodevelopmental change. Recently, using novel time-varying analytic approaches (
88,
89), we found that connectivity measures were differentially related to individual differences in anxiety and depression at different points in adolescent development (
13). A similar approach could be applied to longitudinal neuroimaging and psychotic symptom data, to identify particular periods of development in which psychic symptoms are linked to resting-state fMRI connectivity metrics.
Finally, while we are fairly confident that we were able to appropriately account for site in our analyses (see Figure S4), we observed a statistically significant effect of site in many of the regions of interest (see Table S4). Despite all scans being performed on the same scanner model, there were still differences in task instructions, MRI resolution, and duration of scan. Site effects may have obscured our ability to identify smaller developmental changes in normative amygdala connectivity developmental trajectories. It is also possible that we failed to identify more subtle age-related deviations in amygdala connectivity between the psychosis spectrum and typically developing groups because of site effects. Recently, methodology from genetics has been used to harmonize structural MRI data across sites (
90,
91); modifying and applying this method to resting-state connectivity data is a logical next step. Despite these site differences, we still see a significant interaction between group and age; these findings suggest that multisite neuroimaging data sets will be important for understanding how biomarkers may be sensitive or specific to developmental stage.