In 1998, the first study demonstrating an association between self-reported exposures to abuse, neglect, and family dysfunction before age 18, scores on the Adverse Childhood Experience questionnaire, and a broad range of health outcomes, including cardiovascular disease, diabetes, and depression, was published (
1). Since this seminal study, conducted by the Felitti et al. research group (
1–
4), the Adverse Childhood Experiences questionnaire has been used as a consistent, albeit blunt, predictor of both physical and mental health risks (
2). Within an individual, a higher score on the questionnaire has been associated with substance abuse, suicide, poor perinatal outcomes, and maternal perinatal depression (
2–
5). Given the high prevalence of exposures to adverse childhood experiences (ACEs), their high cross-generational correlation, and their link to maternal mental health, consideration of intergenerational effects is warranted (
6,
7).
Life-course theory suggests that the negative health risks linked to early adversity likely span generations through both direct and indirect pathways (
8). Consistent with this theory, there is evidence of the intergenerational effects of ACE exposures (
9–
12). In previous studies, maternal exposure to childhood maltreatment and trauma was associated with elevated offspring psychopathology in adolescents as well as in children as young as 36 months, often with evidence that maternal factors, including depression, further influenced childhood risk (
13–
18). Beyond maltreatment, studies using broader definitions of early-life adversity provide further support for elevated intergenerational risk. In a recent analysis of the Alberta Pregnancy Outcomes and Nutrition study, maternal scores on the Adverse Childhood Experience questionnaire predicted both maternal depression and externalizing behavioral problems in offspring at 24 months (
19). However, maternal depression only partially mediated the association between maternal Adverse Childhood Experience scores and externalizing behavioral problems, suggesting the influence of other pathways (
19). Whether maternal scores elevate externalizing behavior specifically or psychopathology risk more generally, as well as how early these risk trajectories arise, remains unknown.
Exposure to early-life adversity is hypothesized to result in psychological and biological changes that challenge the adaptive capacity of an individual, putatively resulting in elevated health risk through epigenetic imprints and other mechanisms (
20,
21). One epigenetic factor associated with various characterizations of early-life adversity, including ACEs, is telomere length (TL). Telomeres are highly conserved ribonucleoprotein complexes that cap eukaryotic chromosomes, protecting them from damage and serving as global epigenetic regulators (
22). In the absence of telomere restoration, telomeres shorten with each replication, putatively leading to cellular senescence, apoptosis, or terminal differentiation (
22,
23). TL also influences global transcriptomic regulation, suggesting a more complex role in both stress responsiveness and development (
22,
24). TL is influenced by psychological stress, oxidative stress, genotoxic stress, and neuroendocrine hormones (
25–
29). Two meta-analytic studies have documented the association between early-life adversity and shorter TL within an individual (
30,
31). Individuals with depression and anxiety are reported to have shorter TL, although some debate exists (
32–
36). In a cross-sectional study of adolescents, shorter TL was associated with higher internalizing and externalizing behavioral problems, and TL partially mediated the relationship between parent-child separation and later internalizing and externalizing behavior (
37). Despite correlations between psychopathology and TL, the directionality of the relationship remains unknown. A recent study conducted by Wade et al. (
38) found that, in mid- to late childhood, higher levels of internalizing problems predicted shorter TL during adolescence. Consistent with parental adversity influencing offspring risk of psychopathology, the effects of adversity on TL across generations have been reported. For example, Gotlib et al. (
39) found that daughters of mothers with recurrent depression had shorter TL than daughters of mothers without depression, even in the absence of depression in the daughters themselves. Together, this body of research supports TL as an emerging biomarker capable of capturing both exposure to early-life adversity and heralding future psychopathology risk.
Given the evidence linking ACEs to mental health risk and TL within an individual and evidence of transgenerational transmission of the impact of maternal ACEs, we hypothesized that TL would influence the link between maternal ACEs and childhood socioemotional problems. We first tested the direct effect of maternal ACEs on infant TL across the first 18 months of life. We next examined the relationship between maternal ACEs and infant social-emotional problems at age 18 months. Lastly, we tested how telomere attrition interacted with maternal ACEs to predict externalizing problems while accounting for both maternal postnatal depression and prenatal maternal stress.
Results
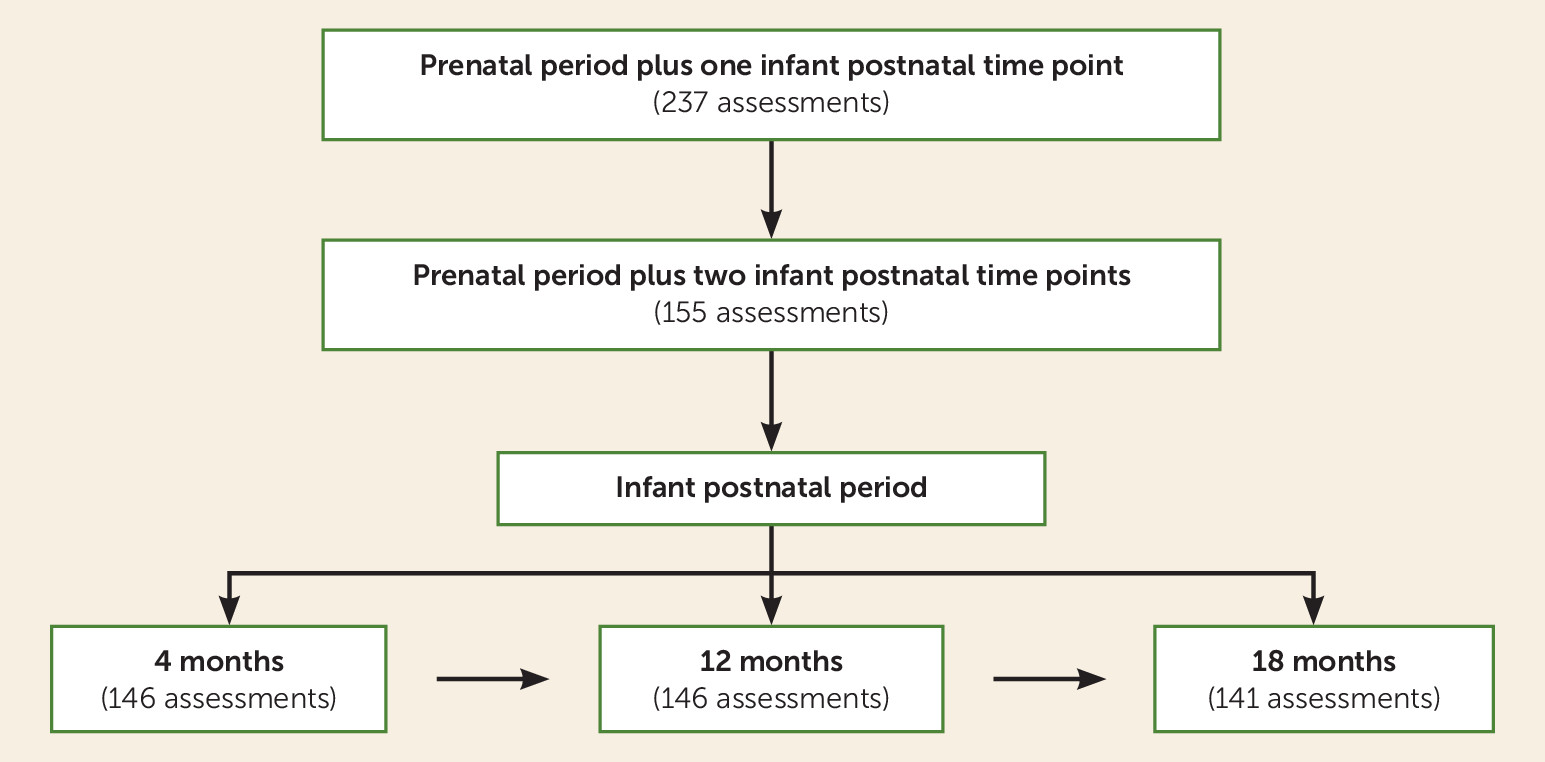
A total of 413 infant TL measurements were obtained from the 155 infants included in the analyses. Overall, 103 infants had TL measurements from all three time points, and 52 infants had measurements from two time points. Of the 52 infants missing one TL measurement, 11 TL measurements were missing at 4 months of age, 22 were missing at 12 months, and 19 were missing at 18 months. Missing TL measurements were either the result of missing one visit (N=31) or infant noncompliance with the buccal swab, resulting in a DNA concentration that was too low for analysis (N=21). Of the 15 imputed TL measurements, 11 were from the 4-month time point and four were from the 18-month time point. Including imputed TL measurements yielded a total of 428 TL measurements for analysis. Infants missing a TL measurement did not differ significantly on any demographic or other outcomes from infants with all TL measurements.
The demographic and clinical characteristics of the study sample are summarized in
Table 1. Correlations between study variables are presented in Table S1 in the
online supplement. The majority of mothers were recruited during the third trimester of pregnancy (N=96, 61.9%), with 34.2% recruited in the second trimester (N=53) and 3.9% in the first trimester (N=6). The mothers’ mean Adverse Childhood Experience questionnaire score was 2.29 (SD=2.08). Higher scores were associated with higher PNMS scores (ρ=0.39, p<0.0001) and lower socioeconomic status (ρ=–0.34, p<0.0001). Maternal Adverse Childhood Experience questionnaire scores were not associated with maternal race (ρ=–0.09, p=0.25). Higher Adverse Childhood Experience questionnaire scores (odds ratio=1.26, 95% CI=1.04, 1.52, p=0.019) and higher PNMS scores (odds ratio=2.10, 95% CI=1.54, 2.86, p<0.0001) were both associated with increased risk for maternal postnatal depression. Twenty-seven mothers (17.4%) were positive for depression at any postnatal visit (
Table 1), 12 mothers (8.3%) were positive for depression when their infant was 4 months old, four (2.7%) were positive when their infant was 12 months old, and 16 (11.4%) were positive when their infant was 18 months old. Mothers who were positive for postnatal depression reported lower socioeconomic status (ρ=–0.30, p=0.0001). Infant TL was significantly correlated across time points. Infants who were racially classified as white or other exhibited shorter TL measurements at 12 months of age compared with black infants (ρ=–0.24, p=0.006), and white infants exhibited a greater rate of telomere attrition compared with black infants (β=–0.249, 95% CI=–0.450, –0.049, p=0.015). No sex differences in TL or telomere attrition were observed.
The mean t score for externalizing problems was 48.57 (SD=11.14, range=28–86) and, for internalizing problems, 44.77 (SD=10.07, range=29–73). With a clinical threshold score of 60, 15.4% (N=21) of infants exhibited clinically relevant externalizing problem scores, and 8.8% (N=12) exhibited clinically relevant internalizing problem scores. Higher Adverse Childhood Experience questionnaire scores were associated with higher externalizing problems (ρ=0.23, p=0.006) but not internalizing problems (ρ=0.16, p=0.070). PNMS score was not associated with externalizing (ρ=0.10, p=0.24) or internalizing (ρ=0.14, p=0.095) problems. Maternal postnatal depression was significantly associated with higher externalizing (ρ=0.19, p=0.031) and internalizing (ρ=0.26, p=0.002) problems. Externalizing and internalizing problems were not associated with telomere attrition or TL at any single time point.
Clustering of TL within each infant (empty model).
The intraclass correlation coefficient of infant TL, using only non-imputed TL measurements, from the empty mixed-effects model was 12.0% (imputed TL intraclass correlation coefficient=9.3%). When accounting for infant age and a nonlinear time factor in the empty model, the intraclass correlation coefficient increased to 18.4% (imputed TL intraclass correlation coefficient=17.3%).
Effect of ACE exposure on infant TL (model 1).
Linear and nonlinear trajectories of TL models were run independently. Including only linear age in the model, TL shortened over time (β=–0.371, 95% CI=–0.468, –0.274, p<0.0001). Including only nonlinear age (i.e., age-squared) in the model, TL also exhibited a significant nonlinear trajectory (β=–0.171, 95% CI=–0.223, –0.119, p<0.0001). The model fit indices for the unadjusted model including linear age, nonlinear age, and maternal Adverse Childhood Experience questionnaire scores were 704.7 and 710.8 (Akaike and Bayesian information criteria, respectively). In the unadjusted model, maternal Adverse Childhood Experience questionnaire scores predicted shorter infant TL across time points (
Table 2) (model 1: β=–0.031, 95% CI=–0.059, –0.003, p=0.033) but did not influence the trajectory of TL over time, as assessed by testing the interaction of maternal Adverse Childhood Experience questionnaire scores and infant age (β=0.014, 95% CI=–0.030, 0.059, p=0.53).
Adjusted effect of maternal ACE exposure on infant TL (adjusted model).
The adjusted model fit indices were 722.6 and 728.7 (Akaike and Bayesian information criteria, respectively). Neither PNMS score (
Table 2) (model 2: β=0.027, 95% CI=–0.029, 0.077, p=0.30) nor maternal postnatal depression (β=0.004, 95% CI=–0.051, 0.183, p=0.96) was associated with infant TL. After adjusting for covariates, higher maternal Adverse Childhood Experience questionnaire scores remained predictive of shorter infant TL (β=–0.039, 95% CI=–0.072, –0.006, p=0.021).
Effect of maternal ACE exposure on infant psychopathology.
Higher maternal Adverse Childhood Experience questionnaire scores predicted higher infant externalizing behavioral problems (
Table 3) (β=1.528, 95% CI=0.562, 2.495, p=0.002) but not internalizing problems (β=0.650, 95% CI=–0.211, 1.511, p=0.14). The model fit indices for externalizing problems were 1,040.51 and 1,063.81 (Akaike and Bayesian information criteria, respectively), and the indices for internalizing problems were 1,009.0 and 1,032.3. PNMS score was not associated with externalizing behavior (β=–0.824, 95% CI=–2.400, 0.753, p=0.31), but maternal postnatal depression exhibited a significant association with externalizing problems (β=6.211, 95% CI=0.978, 11.445, p=0.020). Because the maternal Adverse Childhood Experience questionnaire score was only associated with externalizing problems, internalizing behaviors were not tested in the moderation analyses.
Examination of the interrelationships between maternal ACEs and telomere attrition to predict externalizing behavioral problems.
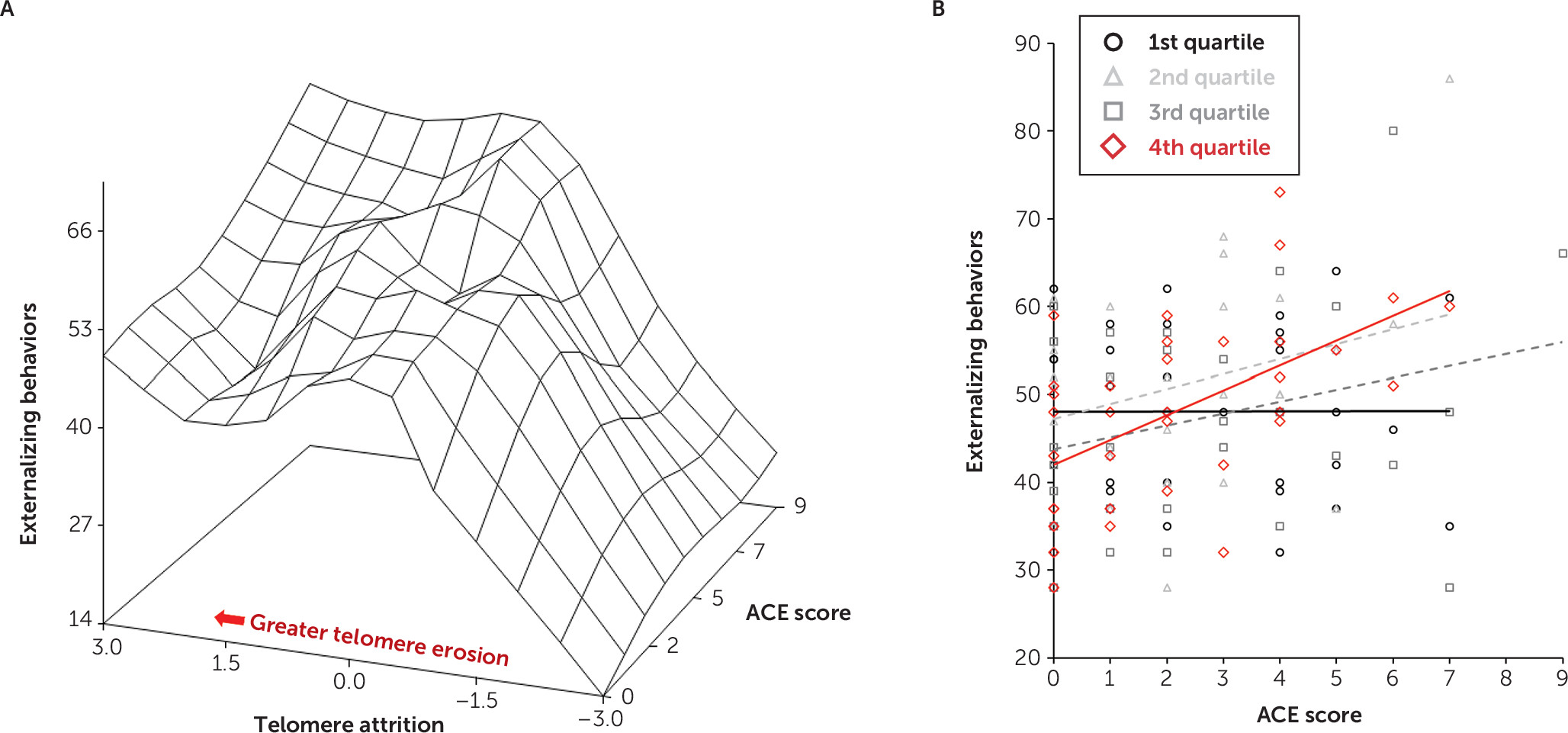
While maternal Adverse Childhood Experience questionnaire scores predicted externalizing behavioral problems and overall TL, telomere attrition did not predict externalizing behavior (β=–0.912, 95% CI=–3.625, 1.802, p=0.51). We therefore tested the effect of the interaction of maternal Adverse Childhood Experience questionnaire scores and telomere attrition on externalizing problems (model fit indices: 1,039.6 and 1,068.8 for Akaike and Bayesian information criteria, respectively). This interaction was statistically significant (
Table 3 and
Figure 2A) (β=1.410, 95% CI=0.110, 2.710, p=0.034). In infants with greater telomere erosion, the relationship between maternal Adverse Childhood Experience questionnaire scores and externalizing problems was strengthened. Maternal postnatal depression persisted as a significant predictor of externalizing problems in this model (β=5.840, 95% CI=0.667, 11.013, p=0.027).
To examine how the magnitude of telomere attrition influenced the relationship between maternal Adverse Childhood Experience questionnaire scores and infant externalizing problems, a quartile split classified telomere attrition in which the first quartile represented the least amount of telomere attrition and the fourth quartile represented the greatest telomere attrition. The model fit indices were 1,044.1 and 1,084.8 (Akaike and Bayesian information criteria, respectively). When compared with the least amount of telomere attrition (i.e., first quartile), the greatest telomere attrition (i.e., fourth quartile) interacted with maternal Adverse Childhood Experience questionnaire scores to predict externalizing behavioral problems, such that higher maternal scores predicted higher externalizing problems (
Figure 2B) (see also Table S2 in the
online supplement) (β=2.902, 95% CI=0.500, 5.305, p=0.018). The fourth quartile of telomere attrition was the only quartile significantly different from the first quartile (see also Table S2 in the
online supplement).
Discussion
This is the first study, to our knowledge, to examine the association between maternal Adverse Childhood Experience questionnaire scores and infant socioemotional problems in the context of a biomarker of cellular stress and aging. Despite being a blunt and retrospective indicator of early-life adversity, maternal Adverse Childhood Experience questionnaire scores predicted both infant TL and externalizing behavioral problems. These results are surprisingly consistent with the existing literature describing the effects of maternal life-course adversity on subsequent generations (
11,
52,
53). Higher maternal Adverse Childhood Experience questionnaire scores were associated with shorter infant TL, a relationship that was not explained by demographic factors, PNMS score, or postnatal maternal depression. These results converge with our previous findings that maternal preconception adversity and PNMS score, despite their correlation, exhibit distinct effects on the next generation (
11,
12) and that alterations in early telomere dynamics contribute to later child socioemotional outcomes (
37,
38). Beyond these findings, the significant relationship between maternal Adverse Childhood Experience questionnaire scores and both PNMS score and postnatal depression warrants additional investigation given their independent implications for maternal and child health.
Early TL and TL trajectories appear to both predict later TL and future health risks (
54,
55). However, to date, only three previous studies (
55-
57), to our knowledge, have examined the trajectory of TL in children, and all three of these studies included only two time points, limiting the ability to detect nonlinear patterns. In the present study of infants ranging between 4 and 18 months of age, TL exhibited an overall linear decrease and a quadratic trajectory. This nonlinear pattern is consistent with the early-life trajectory of leukocyte TL reported for nonhuman primates (
58-
60). These cross-species results suggest that TL exhibits complex trajectories across development, similar to other indicators of growth and aging. Inclusion of multiple TL measurements in longitudinal studies is therefore recommended. The effect of maternal preconception adversity (i.e., ACEs) on children’s early TL trajectories, coupled with the relevance of early TL for future health risks, highlights the need to extend these studies into later childhood and examine both risk and protective factors (
36).
Consistent with the recent study by Letourneau et al. (
19), we found a direct positive association between maternal Adverse Childhood Experience questionnaire scores and externalizing behavioral problems among offspring but no relationship with internalizing behavioral problems. Despite our expectations, PNMS score did not predict externalizing or internalizing behavior or TL. Postnatal depression, however, uniquely predicted both internal and externalizing behavior, even when accounting for maternal Adverse Childhood Experience questionnaire scores. However, maternal postnatal depression did not interact with telomere attrition to predict externalizing problems, suggesting that although maternal postnatal depression is related to infant externalizing behavior and maternal ACEs, the lasting influence on child behavioral problems is not solely transmitted through maternal depression. Despite the strong correlation between maternal Adverse Childhood Experience questionnaire score, PNMS score, and postnatal depression, each appears to demonstrate unique relationships to TL and externalizing and internalizing behavioral problems among offspring. These results complement the few existing studies that implicate complex pathways between maternal life-course adversity and offspring outcomes, providing innovative directions for future research (
61).
Among infants with greater telomere attrition from 4 to 18 months of age, higher maternal Adverse Childhood Experience questionnaire scores were associated with higher externalizing behavioral problems at 18 months. Beyond the influence of maternal postnatal depression, our data indicate that the risk of social-emotional problems as a function of maternal ACEs is compounded by biological processes likely related to TL dynamics, such as inflammation and oxidative stress (
62). Given that a substantial proportion of the variance in externalizing problems remains unexplained, additional biological and psychosocial factors, particularly within the postnatal environment, are expected to further influence transgenerational risks.
There are unique strengths to this study. First, while the Adverse Childhood Experience questionnaire has shortcomings, its established predictive value for health outcomes and its brevity make it an attractive screening instrument for both clinical practice and research (
63). Second, our results extend previous links between maternal early-life adversity and offspring social-emotional problems to an earlier developmental period, highlighting the need for infant mental health screening and intervention programs, especially in high-risk families (
15,
19). Third, this is the largest infant longitudinal TL cohort to date, and it provides evidence, consistent with nonhuman primate studies, of nonlinear patterns of TL trajectory in early development. Finally, this is the first study, to our knowledge, to link maternal preconception adversity to both infant externalizing problems and TL.
Despite the study’s strengths, it has several limitations. While reports associating maternal BMI and TL measured in gestational tissues exist, we did not find an association of maternal prepregnancy BMI with infant TL, and maternal BMI was not associated with internalizing or externalizing problems. Models including maternal BMI did not alter the significance of these findings (
64). We were also only able to partially account for fetal and maternal pregnancy complications. Fetal growth restriction is related to cellular turnover in the placenta and potentially influences cellular turnover and TL in the infant; however, rates of fetal growth restriction were too low for meaningful analyses. Prenatal surveys were conducted at different time points during pregnancy, and we did not collect data on psychotropic medication use or lifetime psychiatric diagnoses; however, adjusting for the trimester of the pregnancy interview did not alter the results. Although we controlled for maternal depression, other factors related to the early caregiving environment, including hostile parenting and the attachment relationship, could influence the relationship between maternal early-life adversity and offspring outcomes. Future studies exploring the effects of early adversity across generations should mirror current efforts to understand the ability of the postnatal environment to moderate the impact of childhood adversity within an individual.
We acknowledge that maternal Adverse Childhood Experience questionnaire scores, PNMS score, depression, and infant externalizing problems were all maternal-reported measures. The differential association between these scores and the collection of data at different time points partially diminishes the concern about bias due to there being a single informant; however, there remains a need for studies that utilize more objective assessments. While reliance on retrospective reports of early-childhood adversity is often cited as a challenge to the body of research using the Adverse Childhood Experience questionnaire, Reuben et al. (
65) found moderate agreement between prospective and retrospective scores and noted that both predicted adult outcomes, albeit with somewhat different relationships. Beyond the issue of reporting bias, the Adverse Childhood Experience questionnaire does not capture the severity, chronicity, or age at which the exposures occurred, since such screening measures, including this questionnaire, must balance the ease of collection with these limitations. Despite these limitations, the predictive capacity of the Adverse Childhood Experience questionnaire score across health outcomes remains remarkably strong.
In addition, the absence of a direct relationship between maternal Adverse Childhood Experience questionnaire scores and infant internalizing behavioral problems was unexpected. Previous studies have suggested that in the toddler age range, the sensitivity of parental reports of child internalizing problems is limited, and, for the most part, internalizing behavioral problems are reported at lower rates than externalizing problems. The use of semistructured interviews (e.g., the Preschool Age Psychiatric Assessment) or other instruments that more specifically target internalizing problems in this age range may clarify whether this relationship is specific to externalizing problems or whether measurement methodology affects the ability to detect relationships with internalizing problems (
66,
67). As a result of the initial study design, accurate measurements of postnatal physical growth (e.g., height, weight, and head circumference) were not obtained at all visits. Although we are not aware of any existing data indicating that infant growth rate from 4 to 18 months is associated with the trajectory of TL, or other markers of cellular aging, this should be considered for future studies, particularly for studies that include low birth weight or preterm infants, because “catch up” growth is associated with elevated cellular turnover and has been linked with shorter islet cell telomeres in rodents (
68-
70). We did not detect sex differences in infant TL, despite evidence in other studies. However, to our knowledge, no studies have reported sex differences in this age range. One potential explanation for this is that sex differences, at least in buccal cell TL measurement, appear later in development. Another consideration, given our findings of racial differences in TL, is that our study was underpowered to examine both sex and race differences, and race may obfuscate sex differences. Disentangling the interactive and independent effects of sex and race in relation to transmission of adversity across generations is an important future research direction.
When considering our measurement of TL, several specific limitations are notable. Changes in TL in buccal epithelial cells may not reflect changes in other cell types, and therefore caution is warranted when interpreting these findings. This remains a challenge to most human studies in which the specific tissue of interest, in this case the brain, is not accessible for study. The use of a change score in the moderation analyses is also a limitation, particularly in light of the nonlinear trajectory of TL. In addition, we did not use a true “baseline” TL measurement because our first TL measure occurred at 4 months of age. Recognizing the increasing importance of baseline TL in understanding the trajectory of TL across the life course, studies capturing a true baseline TL at birth are needed. Because buccal DNA was not available at birth in this cohort, we tested whether TL measured from DNA obtained from newborn blood spots influenced our findings. Despite the correlation between infant buccal TL with bloodspot TL, including bloodspot TL in the models did not influence the results, and we ultimately decided against inclusion in the final analyses because of concerns related to tissue specificity.
In summary, our data, when combined with findings from other studies, confirm maternal life-course experiences as a potent predictor of offspring mental and physical well-being. Too often, studies examine maternal exposures at only one time point (e.g., prenatal or postnatal), and, equally problematic, rely on either biological or behavioral outcomes. Mitigating the lasting effects of maternal adversity on child mental health clearly requires more comprehensive models that are appropriately reflective of the ever-changing interactions between biology, development, and the environment. Our results suggest that screening for maternal ACEs in obstetric, pediatric, and child mental health settings may provide an important indicator of risk for both the mother and the child, especially during infancy. Data from both human and preclinical research provide strong support for the need to integrate a multigenerational life-course perspective into our understanding of the biopsychosocial etiology of mental illness. Encouraging the widespread utilization of practical screening tools that have clinical utility and capture stressors across the life course and the broader environment in which children develop may enhance our ability to understand the origins of early mental illness and the effectiveness, rather than the efficacy, of current intervention and prevention efforts. This same model also offers an unprecedented opportunity for research to capture resiliency factors, such as the early caregiving environment, that may buffer the negative effects of maternal early adversity at the molecular, physiologic, behavioral, and neurocognitive levels.