Posttraumatic stress disorder (PTSD) is a prevalent psychiatric disorder associated with marked occupational and social dysfunction (
1) and characterized by pervasive intrusive thoughts/recollections, avoidance of trauma-related stimuli, hyperarousal, and mood/cognitive impairment (
1–
3). PTSD also has substantial psychiatric and medical comorbidity (
4). Evidence-based PTSD treatments, including psychotherapy and pharmacotherapy, may be less effective in reducing symptoms and improving function in veterans compared with the general population (
5). There is thus a pressing need to develop novel interventions for veterans with PTSD.
Noninvasive neuromodulation is developing rapidly across psychiatry, setting the stage for innovative interventions. A body of literature now supports the efficacy of repetitive transcranial magnetic stimulation (TMS) for pharmacoresistant major depression (e.g.,
6,
7), although this may be less effective in veterans and particularly for those with comorbid PTSD (
8). While existing data suggest the effectiveness of TMS for PTSD (
9–
11; reviewed in
12,
13), we and others (e.g.,
14) believe that lengthy administration times impede broader implementation. Moreover, whether stimulation yields functional improvement remains unknown.
Theta-burst stimulation (TBS) is a novel TMS protocol that rapidly induces synaptic plasticity (
15). During TBS, short bursts of high-frequency (50 Hz) stimulation are repeated at 5 Hz (200 ms interval). TBS can be intermittent (iTBS) or continuous and is associated with long-term potentiation–like and long-term depression–like activity, respectively (
15). Several factors suggest that TBS may be useful in PTSD. First, its brevity allows for easy clinic operation and potential combination with psychotherapy. Second, TBS’s patterned nature resembles theta oscillations of hippocampal memory systems (
16). PTSD is defined, at its core, by the impact of intrusive traumatic memories, and in translational models TBS can induce hippocampal synaptic connections and activity (
17). Taken together, the data provide a theoretical justification for use of TBS for PTSD.
PTSD is associated with pathological function in three large-scale functional networks: the default mode network, the executive control network, and the salience network (
18). The default mode network is involved in self-referential processing and episodic memory; core regions include the medial prefrontal cortex (MPFC), posterior cingulate cortex, medial parietal cortex, and temporal cortex (
19). The executive control network, with hubs in the dorsolateral PFC (DLPFC) and lateral posterior parietal regions, is involved in executive functioning, including emotion regulation and working memory (
20). Finally, the salience network, with hubs in the dorsal anterior cingulate cortex, anterior insula, and amygdala, is implicated in detection of, and attention to, salient environmental stimuli (
21). Neuroimaging studies in PTSD have reported increased salience network connectivity reflecting increased threat detection (
22) and disrupted executive control network connectivity. Reduced default mode network connectivity is generally identified in PTSD (
23–
25, but see also
26), likely related to fear learning and memory dysfunction (reviewed in
27,
28). Network relationships appear important to treatment response, and we previously identified connectivity patterns that predicted PTSD improvement with TMS (
29).
In this study, we conducted the first sham-controlled trial of iTBS for PTSD. We hypothesized that stimulation would be acceptable and efficacious, would reduce symptoms of PTSD, and would improve social and occupational function and quality of life. Furthermore, we hypothesized that efficacy could be predicted using neural networks—specifically, that greater negative (i.e., anticorrelated) connectivity between the salience network/executive control network and default mode network would be associated with improvement in PTSD symptoms.
Methods
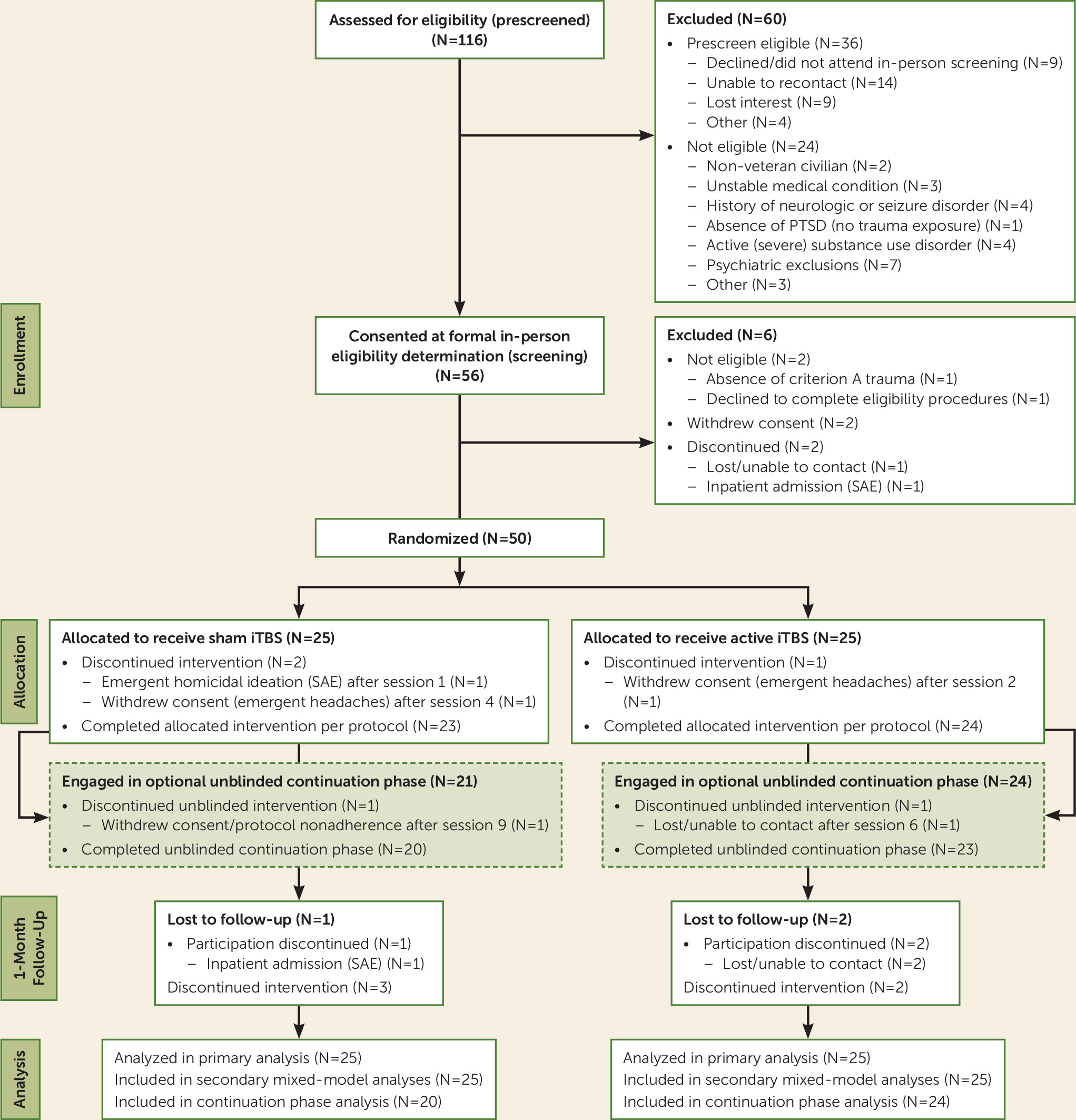
Under institutional review board approval, participants were recruited from the Providence VA Medical Center from May 2016 to December 2017. Fifty-six participants provided written informed consent, and of these, 50 underwent randomized treatment assignment (
Figure 1). The principal inclusion criteria were chronic PTSD by DSM-5 criteria (assessed by the Structured Clinical Interview for DSM-5, which was also used for comorbidity assessment), trauma exposure (assessed using the Life Events Checklist [
30]), age between 18 and 70 years, and, if applicable, being symptomatic despite stable treatment (medications and/or psychotherapy) for at least 6 weeks before undergoing the study procedures. Ongoing treatment was allowed to continue unchanged during the entirety of participation.
Participants were excluded if they had implanted devices (unless MRI safe) or metal above the upper thoracic spine; pregnancy risk; lifetime history of moderate or severe traumatic brain injury (using VA/DoD Clinical Practice Guidelines); current unstable medical conditions; or a history of seizure or other significant neurological disorders, CNS tumors, stroke, or cerebral aneurysm. Other exclusions were primary psychotic disorders, bipolar I disorder, current moderate or severe substance use disorders, or active suicidality.
Randomization and Blinding
Randomization was performed by a study member uninvolved with TBS delivery, in a 1:1 design stratified by PTSD symptom severity and sex. Because the TMS system used separate coils for active and sham stimulation, a staff member uninvolved in treatment delivery or rating scale administration selected the coil for each participant. Blinding was assessed by asking participants to guess their group assignment after the double-blind phase.
Intervention
Using a modified parallel-group double-blind sham-controlled design, we delivered iTBS to the right DLPFC (80% active motor threshold, 1,800 pulses, 9.5 minutes; based on previous TBS studies [
31]). The right DLPFC was chosen because meta-analyses have indicated that right-sided higher-frequency stimulation may yield larger PTSD symptom reduction (
12,
13), and high-frequency TMS administered to this location can reduce amygdala activation to threatening stimuli (
32).
After randomization, active motor threshold was determined (i.e., stimulator output sufficient to induce movement in the contralateral hand >50% of the time), and participants received sham-controlled iTBS daily for 10 business days (intent-to-treat sample, N=25 per group) using a Magstim Rapid 2+1 system (Magstim, Whitland, U.K.). All participants could receive another 10 sessions of unblinded iTBS, to explore the effects of a greater number of iTBS sessions on outcomes. The right DLPFC was found using scalp measurements to place the coil over F4 (modified from reference
33); measurements were checked and monitored at every session to ensure accurate placement.
Statistical Analysis
Our primary outcome measures were acceptability, measured using retention rates and participant reports, followed by changes in PTSD symptoms, using the Clinician-Administered PTSD Scale for DSM-5 (CAPS) (
34). Social and occupational function was measured using the Social and Occupational Functioning Assessment Scale (
35) and quality of life using the Quality of Life Enjoyment and Satisfaction Questionnaire (
36). Blinded raters administered all clinician-administered scales. Self-reported PTSD and depressive symptoms were measured using the PTSD Checklist for DSM-5 (PCL) (
37) and the Inventory of Depressive Symptomatology–Self-Report (IDS-SR) (
38), respectively. Primary clinical outcomes were measured using analysis of variance (
39) to compare groups at the end of the 2-week double-blind phase. Missing data were addressed using multiple imputations (N=20 imputations, or maximum likelihood parameter estimation in mixed models) (
40). Follow-up analyses used a general linear mixed-effect model (
41) with piecewise time effects (
42) to capture the effect of active treatment on outcomes, testing whether active stimulation had superior outcomes compared with sham stimulation up to a 1-month follow-up visit. Linear parameter constraints were used to capture comparable time on active treatment among those in the group initially assigned to active stimulation and those initially assigned to sham stimulation who converted to active stimulation during the unblinded period. This approach permitted inference regarding the effect of cumulative “dose” of iTBS on clinical outcomes observed at 1 month. Statistical analyses were performed in Stata, version 15.1 (StataCorp, College Station, Tex.). Estimated sample size was informed by previous sham-controlled TMS PTSD studies and meta-analyses (
12).
Results are summarized using effect size statistics capturing mean differences standardized to a pooled baseline standard deviation (Cohen’s d) (
43) to reflect estimated change due to treatment (at a particular time point). Data were analyzed in an intent-to-treat fashion, with the intent-to-treat sample defined as participants who underwent randomized treatment assignment and received at least one iTBS session.
Safety
Safety was assessed at every treatment visit by spontaneous adverse event reports coded using the Medical Dictionary for Regulatory Activities, plus a treatment satisfaction form (
44) administered at endpoint. As in previous TMS research, adverse events were analyzed if they occurred in the active treatment group at a rate of 5% or more and at least twice the rate for sham stimulation (
7).
Neuroimaging
Resting-state functional MRI was acquired on a convenience subset of participants (N=26) to identify predictors of improvement. All imaging was obtained less than 5 days before baseline. Neuroimaging data were acquired using a 3-T MRI scanner (Siemens, Erlangen, Germany) and a 32-channel head coil. Imaging acquisition included high-resolution (1 mm
3) anatomical images and 8 minutes of standard resting-state echo-planar imaging. See the
online supplement for details on acquisition, preprocessing, quality control, and motion (used in first- and second-level analyses). MRI data processing used the CONN Functional Toolbox (
45) unless otherwise indicated.
Neuroimaging analyses examined predictors of response to active stimulation. This was based on our mixed-model analysis for clinical outcomes, using clinical change observed during the first 2 weeks in participants initially assigned to active stimulation (N=15) and clinical changes observed when patients initially assigned to sham stimulation received active iTBS (N=11). Because previous research indicated that within- and between-network connectivity predicted clinical improvement in PTSD (
29) and depressive symptoms (
46–
49), we took a focused region-of-interest approach to neuroimaging data analyses. This included a 38-region matrix inclusive of the default mode network, executive control network, salience network, and prefrontal areas implicated in PTSD (described in the
online supplement) to examine connectivity patterns predictive of clinical improvement. Only results that survived false discovery rate correction (
50) are reported; results were robust to post hoc sensitivity tests for data quality and sex unless otherwise stated.
Results
Randomization resulted in groups balanced on demographic variables and symptom severity (
Table 1) that reflected the veteran population. Baseline PTSD scores were in the moderate range. Nearly all (90%) participants met criteria for comorbid depression, and over half had substance use disorders. Baseline ratings also indicated low social/occupational function and poor quality of life. Trauma exposure was multifactorial (see Table S1 in the
online supplement).
Clinical Outcomes
High retention rates indicated acceptability of the treatments; only three participants (two in the sham stimulation group, one in the active stimulation group) withdrew during the double-blind phase (see
Figure 1). Participants were unable to accurately guess their group assignment (χ
2=1.43, p=0.49). At the end of the 2-week double-blind phase, active stimulation compared with sham stimulation produced statistically significant improvement on the Social and Occupational Functioning Assessment Scale (d=0.39, p=0.04). Although active stimulation was not statistically superior on the CAPS (d=−0.12, p=0.61) and the PCL, the effect size on the PCL was clinically meaningful (d=−0.34, p=0.31). We also observed superiority on depressive symptom improvement that approached a medium effect size but was not significant (d=−0.45, p=0.07) (
Table 2).
Mixed-model analyses, which included data from the unblinded phase, found clinically meaningful superiority of active over sham iTBS on most outcome measures (
Table 3). Active stimulation was associated with statistically significant reduction in PTSD on the CAPS (d=−0.74, p<0.001) and PCL (d=−0.63, p<0.001). Superiority was also found on depressive symptoms (d=−0.47, p<0.001) and social and occupational function (d=0.93, p<0.001). Improvements occurred within the first week of active stimulation and were sustained with minimal additional change (i.e., |d| loss/gain <0.2 through follow-up), except on the Social and Occupational Functioning Assessment Scale, which improved over time (
Table 3). We observed statistically significant, but likely clinically irrelevant, improvement on the Quality of Life Enjoyment and Satisfaction Questionnaire (all changes in d <0.1). When we explored outcomes across the two groups, participants initially assigned to active stimulation (i.e., received a total of 4 weeks of stimulation) demonstrated superior results. For example, using a reduction of at least 12 points on the CAPS as the threshold for clinical meaningful results (
51), 67% of those initially assigned to sham stimulation achieved this outcome at 1 month, compared with 81% of those initially assigned to active stimulation (p<0.001, NNT=7).
Safety
Side effects were consistent with previous TMS studies; the most commonly reported adverse events were treatment site discomfort and headache. There were no group differences in reporting (all p values >0.1), although treatment site discomfort occurred more frequently in the active stimulation group (N=6 [24%], compared with none in the sham stimulation group). Three serious adverse events occurred. One participant (who never underwent randomized assignment) could not tolerate MRI procedures, and one participant in the sham stimulation group exhibited emergent homicidal ideation. A third participant, originally assigned to sham stimulation, required hospitalization for suicidality during the follow-up period. There were no seizures.
Neuroimaging Results
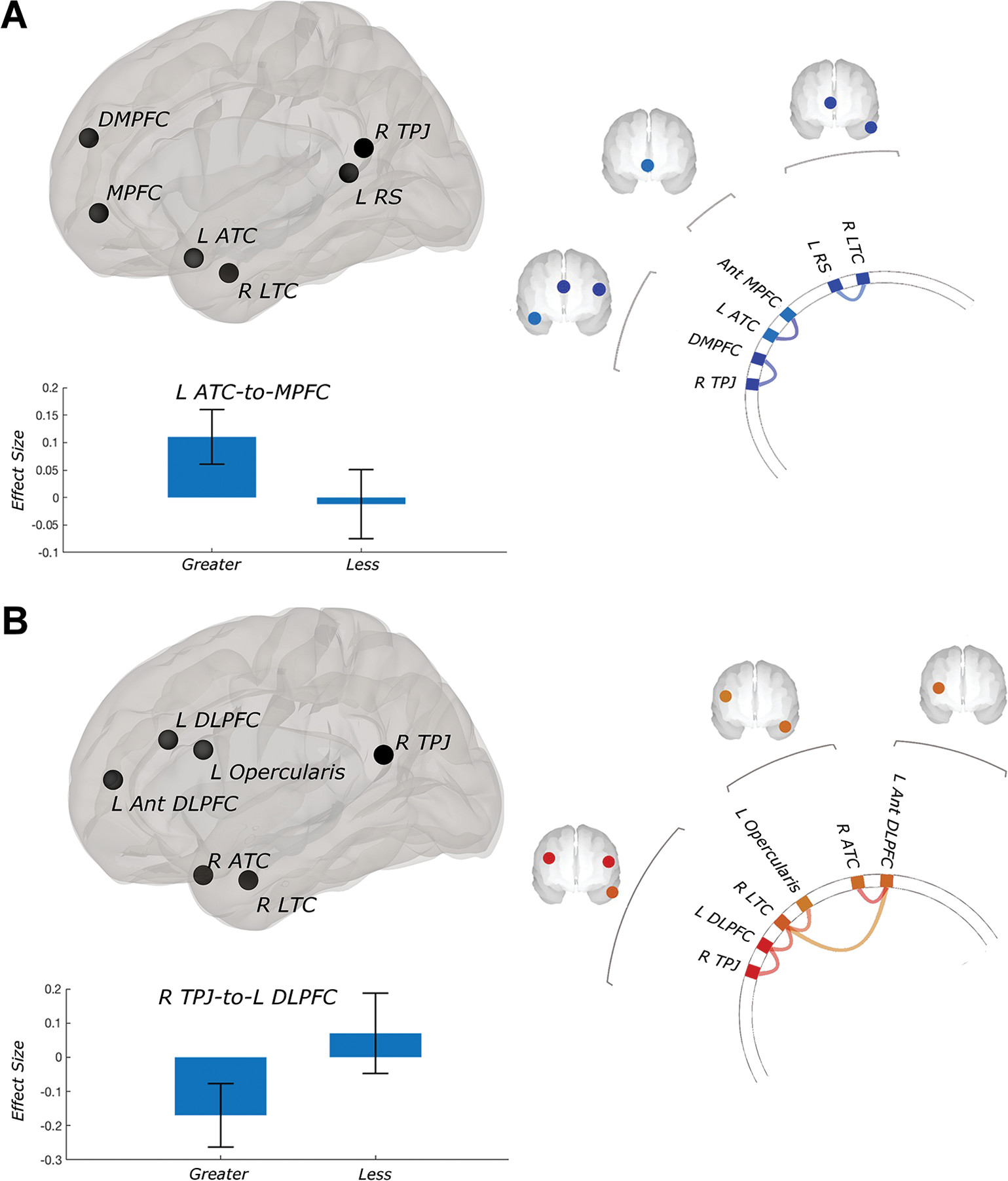
Neuroimaging analyses indicated that baseline resting-state functional connectivity predicted clinical changes with active iTBS. Superior PTSD improvement was associated with stronger within–default mode network functional connectivity (
Figure 2A) and stronger anticorrelated (greater negative) connectivity between the default mode network and externally oriented networks (
Figure 2B).
Significant within–default mode network findings included connectivity between the dorsomedial PFC (DMPFC) and the right temporoparietal junction, and between the MPFC and the left anterior temporal cortex (all false discovery rate–corrected p values <0.05). When we analyzed CAPS scores, the same pattern held but fell short of significance after controlling for data quality (false discovery rate–corrected p=0.06).
Significant cross-network relationships included greater negative connectivity between the left DLPFC (executive control network) and default mode network elements of the temporoparietal junction and anterior temporal cortex, and between the right lateral temporal cortex (default mode network) and left ventrolateral prefrontal cortex/opercularis (all false discovery rate–corrected p values <0.05). These relationships were no longer significant when covarying for sex (false discovery rate–corrected p values >0.1).
Improvement in depressive symptoms was also associated with stronger connectivity within the default mode network–DMPFC network. Connectivity between the right temporoparietal junction and right anterior temporal cortex was associated with improvement (false discovery rate–corrected p<0.01). Depressive symptom improvement was also associated with greater anticorrelated connectivity between the default mode network and the DLPFC, and between the executive control network and the insula (all false discovery rate–corrected p values <0.05). Subgenual anterior cingulate cortex–to–DLPFC connectivity was not predictive of improvement (PTSD or depression), even with lenient statistical thresholds.
Discussion
To our knowledge, this is the first study indicating utility for iTBS in PTSD, with neuroimaging biomarkers predicting clinical improvement. While our results require replication, they are promising. At 2 weeks, active stimulation improved social and occupational function and had indications of efficacy on depressive and self-reported PTSD symptoms; at 1 month (inclusive of data from the unblinded phase), active stimulation was superior across all outcome measures, including self-reported and clinician-reported PTSD symptoms, social and occupational function, and depressive symptoms. Treatment was well tolerated, and side effects were consistent with previous reports. Although one-quarter of participants who received active iTBS reported treatment site discomfort, compared with none in the sham treatment group, headaches were evenly distributed between the treatment groups. Both serious adverse events in the intent-to-treat sample occurred in participants assigned to sham stimulation.
The majority of clinical benefit occurred in the first week of active stimulation. This was unexpected, as previous TMS and iTBS studies in depression indicated that efficacy increased over time with more treatment sessions (e.g.,
7,
52). This suggests that the ideal duration or “dose” of stimulation is an important area of future TBS research; for example, our data suggest that while improvements may occur early, durable clinical effects require greater accumulated exposure to stimulation. We also observed different time courses between depressive and PTSD symptom reduction; depression improved earlier, whereas PTSD improvements were of a larger magnitude by study endpoint. This suggests that future research should evaluate whether change in one symptom domain predicts subsequent change in another and how these trajectories relate to quality of life and functional outcomes.
Neuroimaging analyses demonstrated a role of within- and between-network connectivity in treatment prediction; positive response was associated with increased connectivity within the default mode network and increased negative connectivity between networks. For quality control, we evaluated those participants who received active stimulation only and found comparable results. Within–default mode network connectivity consistently involved memory-related subsystems in the medial temporal lobe. Memory dysfunction is a clinical hallmark of PTSD, and recent research indicates that TMS can modulate memory formation (
53), thus demonstrating the need for further investigation into the interaction between stimulation and memory systems in PTSD.
These results are consistent with a recent report associating greater default mode network functional segregation and reduced PTSD severity (
54), and they are broadly consistent with the idea that patients who have network architecture that more resembles healthy individuals have superior responses to TMS for depression (
48,
55). Our findings, among others, suggest that neural markers of network integrity may be a near-term biomarker of stimulation response, regardless of the technology used or the treated diagnosis. Studies comparing TMS and iTBS (ideally compared with medications and psychotherapy) are needed to identify intervention-specific biomarkers of improvement.
Of note, we did not find that subgenual anterior cingulate–to–DLPFC connectivity predicted clinical improvement. This was unexpected given the comorbid depression in our sample; previous imaging studies of TMS for depression have often, but not always, found subgenual anterior cingulate–DLPFC connectivity to predict TMS response (e.g.,
56; reviewed in
55). Yet we observed conceptually similar results, such that increased negative connectivity between the default mode network and executive control network (i.e., networks inclusive of the subgenual anterior cingulate and DLPFC) predicted superior outcomes.
Parameter selection was guided by the literature at the time of trial design and reflected an intentionally conservative first use of iTBS in PTSD. Recent findings from a study of iTBS for depression using higher motor thresholds (
52) suggested that greater energy may improve efficacy. That study also used a smaller number of pulses per session, reasoning that more stimulation yields an inhibitory effect (at least in the motor cortex) (
57). Whether the use of fewer pulses per session, delivered to nonmotor cortex, has an impact on efficacy remains an important question for future TMS research; the present study should be placed in the context of a wider need for systematic study of stimulation parameters.
Limitations of this study include its modest sample size and the limitations inherent to a demographically homogeneous patient population. Although PTSD in veterans is often associated with combat, our participants reported a wide range of trauma, and all demonstrated comorbid depressive symptoms. While this study demonstrates the use of iTBS in a real-world patient sample, we cannot conclude whether the observed effects are uniquely attributable to stimulation or augmentation of ongoing treatments or whether specific medications or trauma exposure definitively affected outcomes (see the online supplement). Direct comparisons of acceptability of iTBS compared with standard TMS were beyond the scope of this initial iTBS PTSD study. We used scalp-based measurements to place the TMS coil, and neuronavigation might have improved results. Nonspecific clinical effects were likely unequally distributed across the blinded and unblinded phases, favoring unblinded outcomes in mixed-model analyses; longer prospective blinded studies of iTBS are clearly needed. Neuroimaging was obtained in a convenience subset, and we evaluated only regions or networks identified in previous studies, an approach that may have influenced the findings.
In summary, this first study of iTBS for PTSD demonstrated feasibility and preliminary efficacy on clinical symptoms and social and occupational function. Neuroimaging revealed predictors of clinical improvement and underscored the role of default mode network connectivity. This study reflects an important step forward in the use of TMS in disorders other than major depression and demonstrates the broader potential of brain stimulation for patients suffering from psychiatric disorders.
Acknowledgments
The authors gratefully acknowledge the help of Causey Dunlap, B.S., and Marguerite Bowker, R.N., for their assistance with study procedures.