The prevalence rate of depressive disorders is >300 million people worldwide, affecting more than 17 million persons in the United States, and these disorders are a significant cause of years lived with disability (
1). Major depressive disorder is a recurrent, episodic illness, with each episode increasing the risk of future episodes by about 20% per year. Conversely, as the duration of recovery from a depressive episode increases, the risk of recurrence decreases, a phenomenon also observed in bipolar disorder (
2,
3). Among patients with major depression, an estimated one-third have treatment-resistant depression (
4). Typically defined as failure of symptoms to respond to two or more antidepressant trials, treatment-resistant depression carries high personal and societal costs. There is a strong relationship between treatment resistance and increased direct and indirect medical costs, with annual direct costs for patients ranging from $12,000 to $19,000 in the United States (
5). Patients with treatment-resistant depression undergo more medication trials and hospitalizations, have higher rates of disability, and have higher rates of suicide (
6,
7). In the Sequenced Treatment Alternatives to Relieve Depression study, the rate of remission after two failed medication trials was 13.7%. Among those who did achieve remission, two-thirds relapsed within the first year (
8). Among patients with highly treatment-resistant symptoms receiving treatment as usual and observed naturalistically for 2 years, only one-third experienced transient treatment response, and only 4% demonstrated a sustained remission from depression (
9). Identifying treatments that alleviate treatment-resistant depression would have significant individual and societal benefits.
Treatments for treatment-resistant depression continue to evolve. ECT is a highly effective antidepressant treatment, even in patients for whom other interventions have failed, but relapse is common (
10–
12). Transcranial magnetic stimulation (TMS) is approved by the U.S. Food and Drug Administration (FDA) for treatment-resistant depression; however, it is associated with notable relapse among patients who achieve remission (
13–
15). Vagus nerve stimulation, another FDA-approved treatment for treatment-resistant depression, is associated with response rates of 50%−70% over 1–2 years (
16–
18). In a large long-term study, 5-year vagus nerve stimulation outcomes demonstrated a 50% response rate within 1 year—this was about double the rate seen in patients receiving treatment as usual and lasted twice as long (
18). However, patients receiving vagus nerve stimulation have typically shown nonresponse to only 2–4 antidepressant treatments and therefore do not represent patients with the most highly treatment-resistant symptoms (
19).
Deep brain stimulation of the subcallosal cingulate (SCC DBS) white matter has been investigated as a treatment for severe and highly refractory major depression since 2005 (
20). Results have generally been favorable in small open-label trials. With combined results from 193 participants, these studies have shown a response rate of roughly 50%−60% at varying time points (with response defined as a ≥50% decrease in depression severity from baseline). Remission rates (defined by a cutoff on established depression rating scales) were 30%−40% over the same period (
20–
31). Long-term follow-up of the first cohort of 20 patients to receive this treatment showed a sustained treatment response up to 6 years following implantation (
23). Despite these encouraging open-label results, a multicenter randomized sham-controlled trial was halted early because of a lack of statistically significant antidepressant response to active SCC DBS at the a priori 6-month time point. However, the open-label 18- and 24-month outcomes were more promising, demonstrating response rates near 50% (
32). In total, these data suggest a treatment effect that increases and is sustained over time.
Given that patients with treatment-resistant depression are highly susceptible to recurrent depressive episodes, the ability of DBS or any treatment to support long-term maintenance of antidepressant response and prevention of relapse in severe and intractable depression would be an important treatment advance. In this study, we report long-term follow-up data (4–8 years) for 28 patients participating in a single-center open-label clinical trial of SCC DBS for treatment-resistant depression.
Results
Baseline Clinical Characteristics
Twenty-eight participants (19 of them female, 27 of them Caucasian) between the ages of 27 and 65 (mean=45 years) were included in this data set (
Table 1). The first 17 participants (major depressive disorder, N=10; bipolar II disorder, N=7) underwent DBS implantation between 2007 and 2009 in an open-label trial with a 1-month single-blind stimulation-off lead-in period (
22). Between 2011 and 2013, an additional 11 participants with depression underwent implantation using tractography-guided anatomical targeting (
26). A total of 178 patient-years of data were collected and combined for analysis in this long-term follow-up study.
Group Outcomes by Illness Density Index
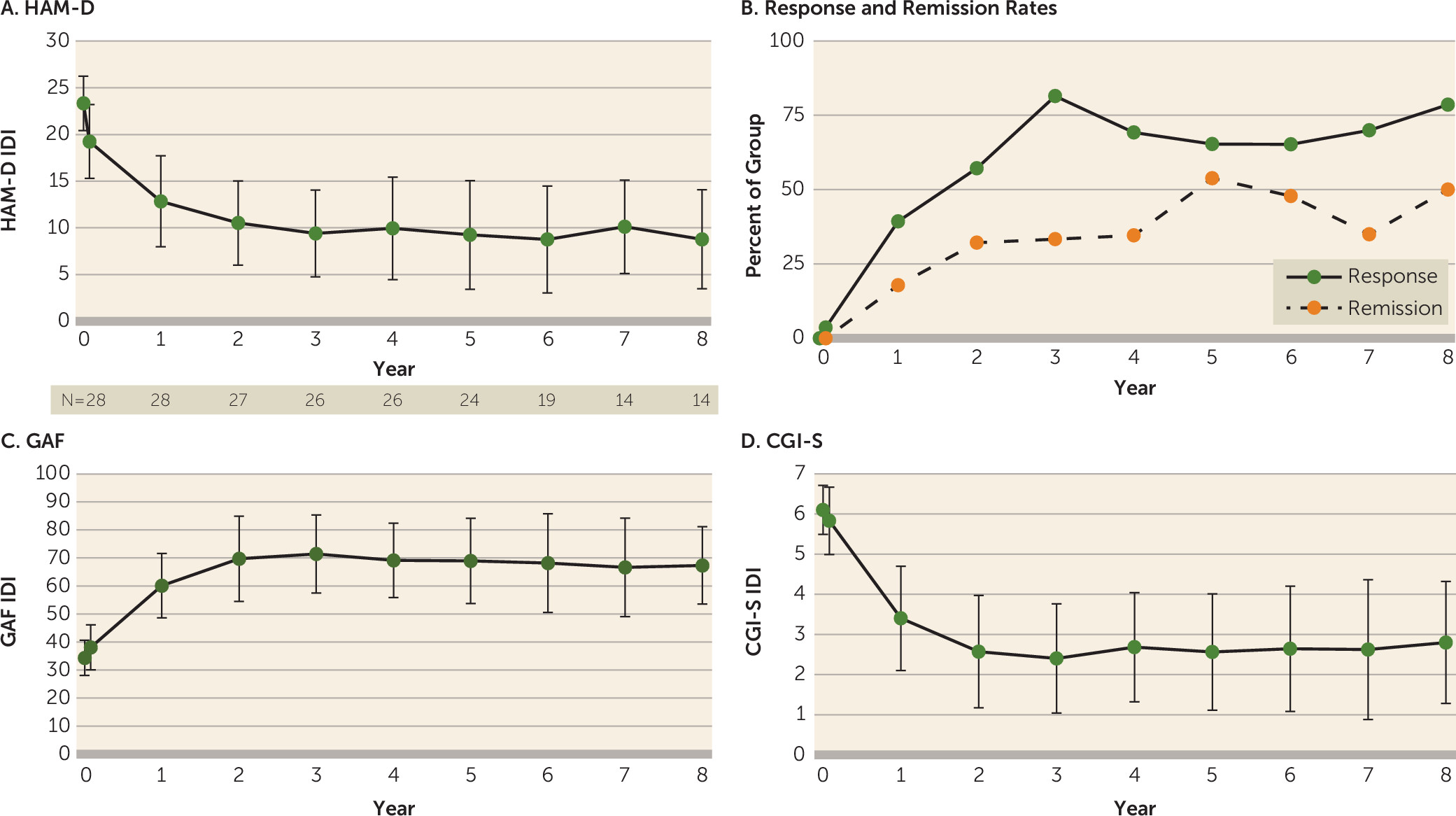
All 28 participants who underwent DBS surgery completed at least 1 year of follow-up. Fourteen participants completed at least 8 years of study participation. As a result of drop-off in the sample size at later time points (primarily because patients had not yet reached those time points), we limited group analysis to the first 8 years of study participation. At year 1, the mean HAM-D score decreased by nearly 50%, and the improvement continued over subsequent years (
Figure 1A). From year 2 onward, the response and remission rates were maintained by at least 50% and 30%, respectively (
Figure 1B). The mean GAF score at baseline was 34.4 (SD=6.1) (indicating major impairment in several areas) and improved to the 61–70 range (indicating mild symptoms but with overall good functioning) in years 2–8. Similarly, the mean CGI severity score improved from 6.1 (SD=0.6) (severely ill) at baseline to <3 (mildly ill or better) in years 2–8.
Sustainability of Response in Participants
Twenty participants (71%) demonstrated consistent improvement of ≥25% from baseline depression severity ratings throughout the study (see Figure S1 in the online supplement). Eighteen participants (64%) showed improvement of ≥50% from baseline for the majority of their years of participation in the study. Among these long-term responders, 13 (46%) demonstrated a continuous antidepressant response after first reaching study response criteria. Six participants (21%) maintained ≥50% improvement continuously since their first year of participation. One participant with a robust and sustained response experienced a return of depressive symptoms only in the setting of device failure (see Figure S1 in the online supplement, patient 14).
Response Patterns in the Bipolar Disorder Subgroup
Seven participants with bipolar II disorder were enrolled in this study, with one additional participant having a diagnosis of major depression reclassified as bipolar II disorder after a hypomanic episode that developed several years after inclusion in this study. These eight participants with bipolar disorder had an average of 12.4 lifetime depressive episodes and 7.6 hospitalizations. Patients with bipolar disorder had a significantly shorter duration of current depressive episodes compared with patients with depression (
Table 1). Among the eight patients with bipolar disorder, five showed a favorable response pattern, and three exhibited limited antidepressant response over time. Four patients in the bipolar disorder group withdrew from the study, accounting for four of the five participants who withdrew from the study overall.
Safety
There were 56 serious adverse events across 178 patient-years (
Table 2). Of these, 10 occurred in a single patient whose DBS device was explanted after 2 years of participation (see Figure S1 in the
online supplement, patient 5). There were 19 surgery-related serious adverse events, including six infections in four patients, with half related to the initial surgery and half related to an implantable pulse generator replacement. Six participants underwent reimplantation of intracranial DBS leads after required explantation as a result of an infection compromising connecting wires (N=3) or a device malfunction (N=2) or to improve targeting (N=1). One patient experienced a small intraoperative cortical hemorrhage and postoperative seizure, with no focal neurologic symptoms or sequelae, and no subsequent treatment for seizures. There were 15 device-related serious adverse events, primarily as a result of device malfunction, such as extension cable breaks. There were 21 psychiatric adverse events, with 20 associated psychiatric hospitalizations (
Table 2). Seven of these adverse events and hospitalizations were for a single participant. Six serious adverse events and hospitalizations were as a result of anxiety. Five adverse events included suicide attempts among three participants. Two of three suicide attempts occurred in a single participant during a period of symptomatic remission (see Figure S1 in the
online supplement, patient 13). There were no instances of stimulation-induced hypomania or mania. All except four hospitalizations and three suicide attempts occurred before patients met criteria for treatment response. There were no suicides.
Clinical Outcomes
Currently, 23 patients continue in long-term follow-up. Five participants withdrew from the study after 1, 2, 5, 8, and 11 years, respectively. Of these, two exhibited no significant symptom improvement during the study (see Figure S1 in the online supplement, patients 6 and 15). One patient who withdrew exhibited significant improvement in depression symptoms (see Figure S1 in the online supplement, patient 5) but was lost to follow-up and was removed from the study for protocol noncompliance. That participant later had the DBS device explanted on request, but symptom severity was not documented at that time. Two participants demonstrated antidepressant response with stimulation but nevertheless elected to withdraw from the study (see Figure S1 in the online supplement, patients 2 and 9). Of the five participants who withdrew, one (patient 2) declined to have the device explanted and continues to be treated at our facility and has not had a significant change in mood symptoms since withdrawing from the study.
For the participant (described above) originally diagnosed with major depressive disorder but subsequently diagnosed with bipolar II disorder after a hypomanic episode that emerged >3 years after start of DBS (see Figure S1 in the online supplement, patient 11), the hypomanic episode was associated with psychotropic medication changes but was not associated with DBS parameter changes and did not require DBS treatment parameter modification or interruption. The hypomanic episode resolved with additional medication changes (but no changes in DBS parameters).
Stimulation Parameters
All participants were started on a single contact per hemisphere with monopolar stimulation at 6 mA. Stimulation remained constant in frequency (130 Hz) and pulse width (91 and 87 microseconds for the Libra XP and Brio implantable pulse generators, respectively). The current was the only variable modified, with all participants receiving between 5 and 9 mA (6–8 mA for 21 of the participants). There were 24 contact changes in 12 participants, and no contact changes were made after 1.5 years of chronic stimulation. Most contact changes were made in the earlier participants before the implementation of prospective tractography for target selection.
Device Considerations
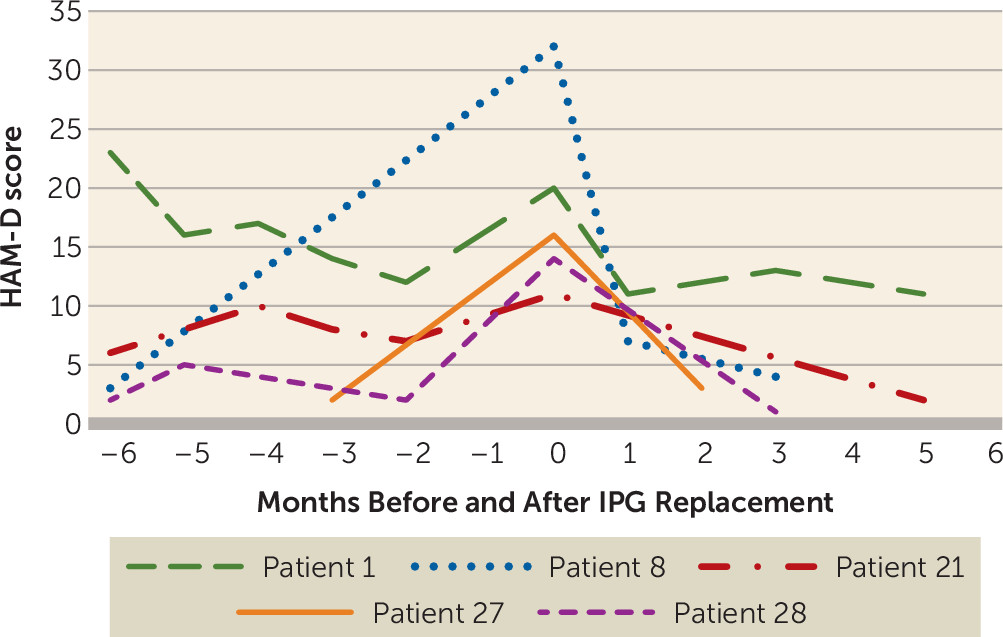
The average life of a nonrechargeable Libra XP implantable pulse generator in this study was 16.5 months (SD=3.3). Each participant underwent two to six implantable pulse generator replacement surgeries. While not systematically assessed, it was observed that a subset of patients exhibited a clear gradual worsening of depressive symptoms with battery depletion. Hardware replacement surgery and resumed stimulation recaptured the previous level of response in all patients, typically over a period of weeks (
Figure 2). Two patients (one responder, one nonresponder), each several years into their study participation, chose not to schedule an implantable pulse generator replacement when their battery was depleted (see Figure S1 in the
online supplement, patients 1 and 13). Both of these patients experienced subjective return or worsening of their depressive symptoms over time and ultimately underwent implantable pulse generator replacement and returned to their previous level of improvement after stimulation was resumed.
Long-Term Clinical Management
One participant started the study while not taking any antidepressants and has remained a continuous responder. However, in the subsequent years after study entry, this patient started taking one antidepressant and then a second antidepressant to address residual symptoms. At least nine participants (all with major depression) who experienced a period of wellness attempted to decrease or discontinue their medications. Two participants successfully discontinued all psychiatric medications and have maintained remission (see Figure S1 in the online supplement, patients 17 and 21). However, most of the patients who attempted to decrease or discontinue medications were unable to tolerate total discontinuation, and they continue to take one or more antidepressant medications.
After the first 6 months of DBS, participants returned to their local psychiatrists for routine clinical care. Some participants undergoing stimulation treatment experienced worsening of depressive symptoms at times (sometimes captured at study visits, sometimes not). Once optimal stimulation settings were established (best contact verified with tractography, stable stimulation parameters), further management of psychiatric symptoms involved traditional approaches (i.e., medications or psychotherapy). Stimulation adjustments were rarely made. No participant was treated with ECT after enrolling in the study.
Discussion
The results presented here support the long-term safety and sustained efficacy of SCC DBS in an open-label long-term follow-up study of participants enrolled in a clinical trial of SCC DBS for severe treatment-resistant depression, including patients with bipolar II disorder. No adverse events were attributable to acute or chronic stimulation, including a lack of stimulation-induced mania or hypomania (even in participants with bipolar disorder). There were five suicide attempts over 178 patient-years of observation, or about three attempts per 100 patient-years. A recent meta-analysis reported a rate of 4.66 suicide attempts per 100 patient-years in patients with treatment-resistant depression (
7). Although a direct comparison between patients in this meta-analysis and our relatively small sample is not possible, it is noteworthy that the average age and gender ratio of patients in the two studies are similar, and follow-up in our study is longer than the average follow-up across studies reviewed in the meta-analysis (about 2 years).
Surgical treatment of a psychiatric disorder, particularly one with permanently indwelling hardware, will necessarily be associated with more medical and surgical complications than traditional psychiatric treatments. In our study, there were 19 serious adverse events related to surgery, or 0.7 events per participant or 0.1 per patient-year. In comparison, the rate of serious adverse events across several prospective multisite trials of DBS for neurological indications averaged 0.5 serious adverse events per participant (range, 0.125–1.12) (
34). The 15 device-related serious adverse events in the present study equate to a rate of 4.2% serious hardware complications per electrode-year, compared with 8.4% in an earlier study of hardware complications in DBS for other neurological disorders (
35). Thus, while medical complications, such as infection and device malfunction, are higher in DBS for treatment-resistant depression than would be observed with traditional psychiatric treatments, the medical complication rate in the present study is comparable to that seen with DBS for other indications.
These open-label, long-term data are consistent with earlier findings by Kennedy et. al. (
23), who reported 4-year open-label follow-up results of another cohort of patients with treatment-resistant depression receiving SCC DBS. In the present study, the group mean HAM-D score, calculated using the IDI, was near 10 from the second year onward, down from a baseline score >23. Antidepressant response rates were maintained at or above 50%, and remission rates were maintained at or above 30%. In tandem with this, global illness severity decreased, while global functioning increased. However, it should be emphasized that the absence of a long-term control group (e.g., treatment as usual, blinded discontinuation, or long-term sham treatment) precludes firm conclusions regarding the role of chronic DBS in these long-term outcomes.
Reporting group means over time can obscure individual patterns of responsiveness to treatment or expectable fluctuations in mood or disease course over years. We believe that yearly IDI measures, while inevitably smoothing some of the fluctuations from within-year mood ratings, more accurately represent the year-to-year experience of depression in individual patients with lifelong mood disorders. It allows for utilization of all available data over time, rather than relying on data from a single, potentially arbitrary time point. Individual response patterns in this group of patients, who presented at baseline with a depressive episode averaging >4 years in duration, show that nearly two-thirds of these patients demonstrated years of sustained, significant symptom improvement after DBS, with many showing sustained remission from depression. Specifically, the persistence of the DBS treatment effect at both the group and individual levels is remarkable and is unmatched by other treatment modalities in patients with symptoms with this degree of treatment resistance, who often experience nonresponse or loss of response to ECT. Vagus nerve stimulation studies have similarly reported a slower onset and fairly well maintained antidepressant response. In a 5-year study of vagus nerve stimulation compared with treatment as usual, the median time to first response and median time to first remission were 12 months and 49 months, respectively, in the vagus nerve stimulation group, compared with 48 months and 65 months, respectively, in the treatment as usual group (
18). The median time to recurrence of depression among remitted patients was 40 months in the vagus nerve stimulation group, compared with 19 months in the treatment as usual group. Studies of the maintenance of TMS response have rarely assessed patients beyond 6 months, but these studies have shown a continued response rate of 52%−62% at the 6-month time point (
15,
36). Long-term psychotherapy (18 months) for treatment-resistant depression compared with treatment as usual showed limited remission after 42 months of follow-up (15% in the psychotherapy group compared with 4% in treatment as usual), but response rates of 30% compared with 4.4% were observed after 42 months of follow-up (
37).
The necessity of ongoing DBS to maintain long-term clinical remission remains untested, because blinded discontinuation was not repeated after the first three study subjects (
22). Anecdotal evidence here, as well as that reported by others (
23,
27,
28), suggests a return of depressive symptoms after planned or unplanned withdrawal of stimulation (e.g., as a result of hardware malfunction or battery depletion), with return of clinical gains after resumption of stimulation. Puigdemont et al. (
38) examined the SCC DBS target and found similar loss of antidepressant effects with controlled discontinuation. Across these various studies, depressive symptoms typically returned in the absence of stimulation, even after several years of chronic stimulation. Future studies will need to test these observations by discontinuing stimulation under controlled conditions, to address questions of DBS efficacy as well as safety risks.
Over several years of observation and clinical management, participants in this study required little additional clinical management beyond standard psychiatric follow-up and intervention. Most participants were principally observed by their local psychiatrist. The study team met with the patients twice yearly. After determining the best stimulation contacts early in the treatment course, additional changes to stimulation parameters were rarely made. Most of the additional care required for SCC DBS patients beyond standard psychiatric care is related to battery replacement, a burden that should be eased with the introduction of longer-lasting or rechargeable battery systems. Most participants continued to take antidepressant medication to support their mood. It is unknown whether the mechanism of SCC DBS inherently requires adjunctive medication treatment or whether its continued efficacy is predicated on the brain milieu at the time stimulation is initiated.
Identifying factors associated with long-term response is an important next step in SCC DBS research. Positive outcomes would seem to be dependent on pairing optimal procedural accuracy (e.g., anatomic targeting, stimulation parameter selection) in patients with an underlying biology responsive to this intervention. It has been observed that melancholic features and previous periods of functional recovery from depressive episodes appear to be associated with better DBS outcomes (
39). Most participants in our study met criteria for the melancholic depression subtype. However, we were unable to empirically confirm the relationship between response and previous recovery periods with available data. Future studies would benefit from careful documentation of the degree of interepisode recovery. Beyond this, more qualitative studies of DBS patient characteristics and the quality of patients’ experience of depression before and after DBS are needed in order to optimize patient selection for DBS in the SCC and other brain targets.
Larger blinded, controlled trials will be necessary to validate the efficacy and safety of SCC DBS. The first such trial failed to show a statistically significant difference between active (17%) and sham (22%) stimulation at the predefined 6-month endpoint, but a progressive increased response rate of 53% and 49% with open-label stimulation at 18 and 24 months, respectively, was observed (
30). Taken together, these results support a reassessment of clinical trial designs for studies of DBS for treatment-resistant depression, in order to conduct trials that will assess not only short-term efficacy but meaningful, sustained response over the long term.