Anxiety is “a future-oriented mood state associated with preparation for possible, upcoming negative events” (
4) and is usually a normal and adaptive behavioral response to everyday life. In anxiety disorders, anxiety is excessive or out of proportion to the actual or anticipated event and is accompanied by clinically significant distress or disability (
5). Numerous risk factors for anxiety disorders have been studied, including experiential and genetic factors (
6). For example, neurotic personality traits are predictive of the onset of anxiety disorders (
7). Twin studies demonstrate a heritable component to anxiety disorders (
6), but there have been few published genome-wide association studies (GWASs) to date investigating anxiety or anxiety-related traits. The Anxiety Neuro Genetics Study (ANGST) (
8) meta-analysis was the first large GWAS to identify significant genetic associations, finding one genome-wide significant locus each for a categorical case-control design for any anxiety disorder diagnosis and a quantitative factor score for anxiety in a cohort of over 18,000 subjects. Another recent large GWAS, from the Lundbeck Foundation Initiative for Integrative Psychiatric Research (iPSYCH), identified a significant genetic association with anxiety and stress-related disorders in a cohort of 31,880 individuals in the national Danish registers (
9). Also of note is a study based on the UK Biobank cohort, the second largest GWAS of anxiety to date (
10), which examined anxiety using case-control and clinical cutoffs based on a score ≥10 on the Generalized Anxiety Disorder 7-item scale (GAD-7). While progress is being made, understanding of the genetics of anxiety disorders has lagged behind other related disorders, such as major depression (
11).
Only a third of individuals with anxiety disorders receive treatment (
12). For those who do enter treatment, psychological approaches such as cognitive-behavioral therapy (CBT) have been shown to be effective (
13), as have certain pharmacotherapies (
14). A recent systematic review of CBT treatment response rates for anxiety disorders showed average rates of 49.5% at end of treatment and 53.6% at follow-up (
15). A better understanding of genetic risk factors and determinants now informs other aspects of medicine, such as oncology and cardiology, through identification of causal mutations (
16) and variants, and this approach will have important implications for psychiatry (
17). These precision-medicine approaches are challenging in complex traits such as anxiety, which are associated with many (perhaps hundreds of thousands of) variants of individually small effect (
18). The use of polygenic risk scores will require a suitably large sample size to provide sufficient confidence in these small individual effects that cumulatively account for so much of the heritability (
19). Underlying polygenic risk factors from sufficiently large cohorts may inform an approach to identifying individuals with a predisposition to anxiety disorders and to improving outcomes.
Discussion
We present the largest GWAS to date for anxiety traits, employing a quantitative phenotype, the GAD-2 score, in nearly 200,000 MVP subjects, as well as self-reported physician diagnosis of anxiety/panic case-control phenotypes in >220,000 MVP subjects. We identified novel genetic variants in and around several genes, some of which have previously known functional relationships with anxiety. These genes play roles in the hypothalamic-pituitary-adrenal (HPA) axis, neuronal development, and global regulation of gene expression.
There is high comorbidity between anxiety, PTSD, and depression. We used a PRS derived from the MVP GAD-2 analysis to identify genetic overlap with the independent PGC PTSD and major depressive disorder GWASs (
Table 3; see also Figure S7 in the
online supplement). We found significant genetic overlap between these traits, providing biological evidence that this known clinical comorbidity is due at least in part to shared genetic etiology. Additionally, we performed multi-trait-based conditional and joint analysis, using a prior GWAS of depression to condition the results of the present GAD-2 GWAS. In this analysis, we show not only that the peak signals for anxiety are reduced in magnitude (see Figure S3 in the
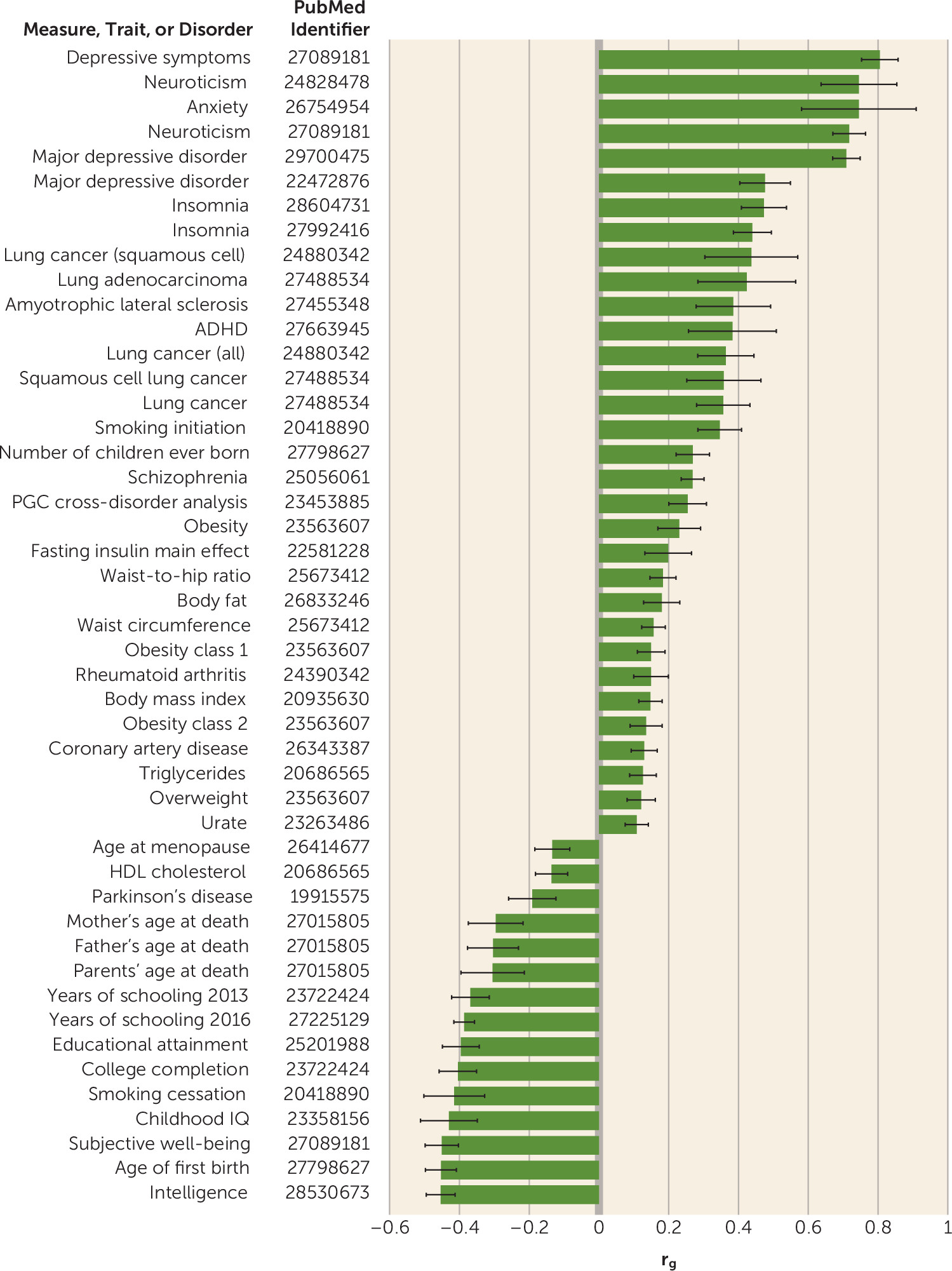
online supplement) but also that the overall heritability for anxiety symptoms is diminished, from 5.58% to 4.29%, when conditioned on genetic liability to depression. Via linkage disequilibrium score regression, we identified substantial genetic correlations between anxiety and numerous other traits (
Figure 2). Particularly noteworthy were positive correlations with depression and neuroticism as well as a negative correlation with subjective well-being (
Figure 2). These findings replicate similar correlations found using a case-control approach (
9).
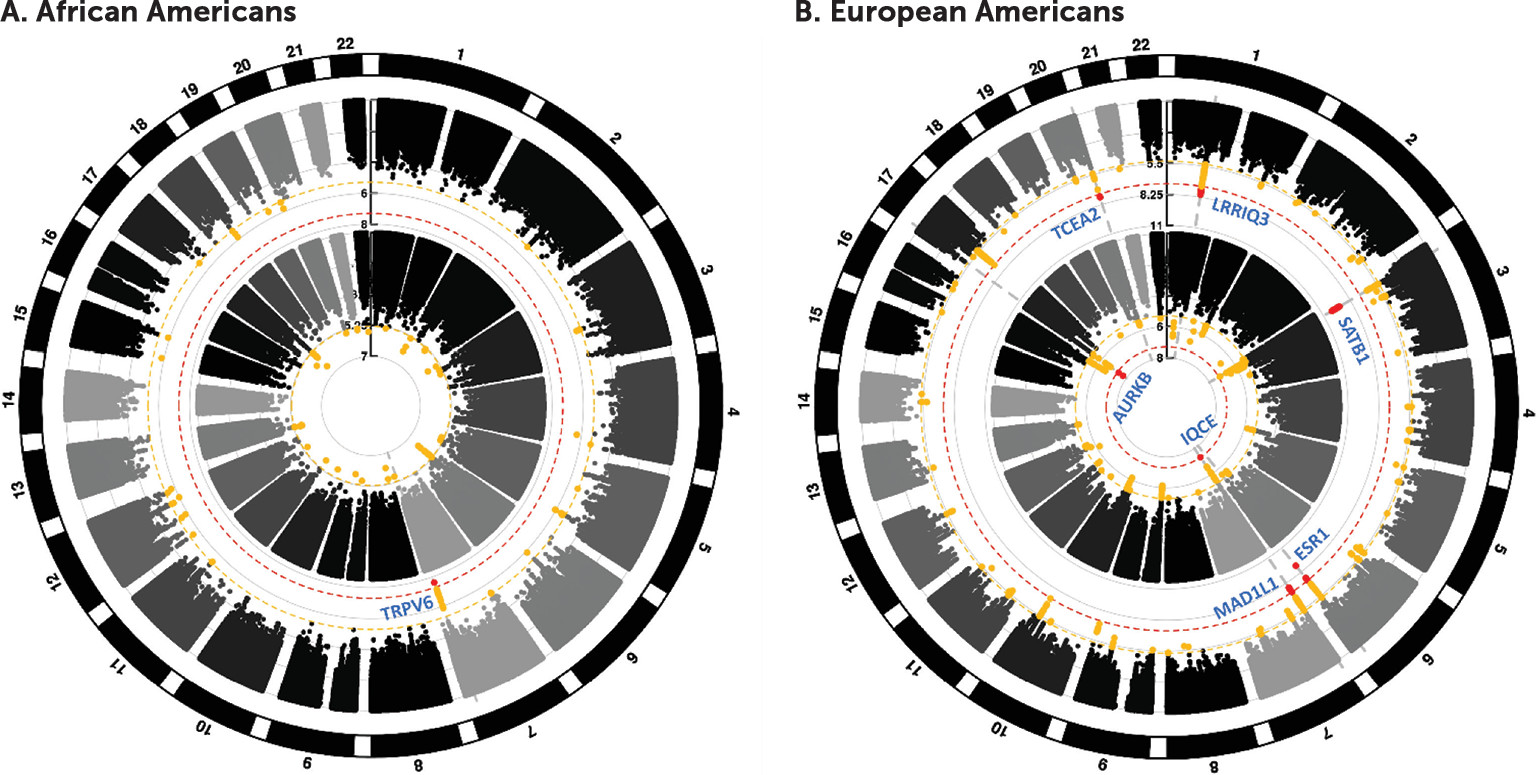
The genome-wide significant result in African Americans is an insertion variant that is rare outside of African ancestry and occurs in a genomic region proposed to be under recent selection in Europeans (
36). The lead SNP is at
TRPV6, which encodes a Ca
2+-selective membrane cation channel associated with epithelial calcium transport and homeostasis in kidney and intestine. The lead SNP rs575403075 has an MAF range of between 0% and 1% in non-African populations and would fall below MAF quality control thresholds used for common variants in most non-African populations. In individuals of African ancestry, this variant is much more common, with an MAF in our study of 5.8%. This highlights the importance of studying genetic risks in diverse populations—otherwise these signals may be missed entirely.
The top genome-wide significant findings for European Americans in the GAD-2 analysis were in and around
SATB1 and the antisense gene
SATB1-AS1.
SATB1 is a global regulator that influences expression of multiple genes involved in neuronal development (
37). One gene modulated in expression is Corticotropin Releasing Hormone (
CRH), encoding the protein product of the same name that plays an essential role in the HPA axis, which has frequently been shown to modulate stress and fear/anxiety response (
38). The
CRHR1 (Corticotropin Releasing Hormone Receptor 1) gene was genome-wide significant in the gene-based association analysis (p=3.60×10
−7).
CRHR1 has been a proposed target for treatment for anxiety and stress-related disorders, with evidence for anxiolytic-like effects of CRHR1 antagonists in animal models although not yet in humans. Based on our findings, we speculate that individuals with differing genetic risk that does or does not involve this pathway may differ in their responses to CRHR1-targeted and other glucocorticoid-targeted therapeutic agents; this may be a reasonable pathway to address via personalized medicine, and it presents a testable hypothesis.
The estrogen receptor
ESR1 (also known as estrogen receptor alpha) has been a focus in animal models of anxiety-like behaviors, and these have provided mechanistic validity for the role of
ESR1. Studies of estradiol administration to ovariectomized rats and ESR1 null mice have shown consistent evidence that
ESR1 is involved in anxiety-like behavior (
39). Our finding of an association between
ESR1 and anxiety may have implications for our understanding of sex differences in anxiety disorders and trauma and stressor-related disorders such as PTSD, which are more common in females (
40). Although this female predominance is partially explained by sex-specific exposure to certain kinds of traumatic events (e.g., domestic violence, sexual assault), there may also be differential biological context provided in part by the role of the estrogen receptor. Our study in a predominantly male sample identifies
ESR1 as genome-wide significant. Estrogen is important in both sexes, and a recent review has highlighted the important role for estrogens in men (
41). Studies with larger numbers of women will be needed to more fully investigate sex differences in genetic risk for anxiety-related traits.
Previous genetic epidemiology studies have shown that common genetic factors can underlie anxiety and depressive traits (
42). The lead SNP from the GAD-2 GWAS near the
LINC01360 and
LRRIQ3 (rs2180945) loci is nominally significant and has the same direction of effect in the 2018 PGC major depressive disorder analysis (p=1.434×10
−6) (
11). This variant may be linked to a common risk factor for both disorders.
One genome-wide significant signal for GAD-2 was in a gene-rich region on chromosome 20 near
TCEA2,
C20orf201,
RGS19, and
OPRL1, with fine-mapping analysis prioritizing a causal region in the latter gene.
OPRLI (which encodes the amygdala nociceptin/orphanin FQ receptor) is involved in learning and memory and anxiety and fear-related behaviors (
43,
44) and has been hypothesized to play a role in anxiety and stressor-related disorders such as PTSD (
44). Interestingly, fear conditioning was also significantly enriched in the pathway analysis. Taken together, these observations suggest that
OPRL1 and related systems should be further explored as targets for anxiety and stressor-related therapeutics. eQTL data suggest that variants in this region regulate expression of
RGS19 and
OPRL1 in the cerebellum and in the basal ganglia (see Table S7 in the
online supplement). The basal ganglia have long been implicated in obsessive-compulsive disorder and anxiety disorders. A recent review discussed cerebellum-linked neurocircuitry to anxiety and fear behaviors in rodents and in humans (
45). The cerebellum is thought to play an important role in anticipation/prediction processes. Given that anxiety has been defined as “a future-oriented mood state associated with preparation for possible, upcoming negative events” (
4), these results may provide further evidence for a role for the cerebellum in fear and anxiety.
MAD1L1 (GAD-2 lead SNP rs56226325, MAF=0.17, p=2.01×10
−8; self-reported physician diagnosis of an anxiety disorder lead SNP rs10534613, MAF=0.41, p=4.92×10
−8) is replicated in the iPSYCH anxiety GWAS data (
9) (
Table 2; p=6.85×10
−4) and has been associated previously with bipolar disorder (
46). One of the lead SNPs in the iPSYCH study is also nominally associated with anxiety in the present study (rs11764590, p=3.36×10
−7); this SNP is in LD with our lead SNP, rs56226325 (r
2=0.69). A recent large GWAS of bipolar disorder identified genome-wide significant SNPs in the
MAD1L1 locus, although their lead signal is not significant in our study of anxiety (rs4236274, p=0.27) (
47). This locus has also been identified among 108 genome-wide significant loci by the PGC schizophrenia study (rs58120505, p value=6.43×10
−14) (
48), and our lead SNP is nominally significant in that study (rs56226325, p=1.12×10
−3). This SNP is also nominally significant (6.71×10
−4) in the 2018 PGC depression GWAS (
11). Taken together, these observations suggest that this locus may be a common risk factor for several psychiatric disorders.
MAD1L1*rs56226325 is also an eQTL for expression of
FTSJ2 (see Table S7 in the
online supplement), and this variant is associated with decreased expression in the brain. MAD1L1 is a mitochondrial RNA methyltransferase that is important for the proper assembly of the mitochondrial ribosome and cellular respiration (
49). The protein product of
FTSJ2 is Mitochondrial rRNA Methyltransferase 2 (MRM2), which was implicated in a case study of a 7-year-old Italian boy with a damaging mutation that reduced the catalytic activity of
MRM2, leading to an encephalopathy, lactic acidosis, and stroke-like (MELAS) syndrome (
50). Larger-effect mutations at this locus can have devastating effects on the brain; smaller-effect variations may be less deleterious but still cumulatively influence development, which may predispose to neurological and psychiatric disorders.
The Brainstorm Consortium has investigated shared heritability between psychiatric and neurological disorders (
51). Consistent with their findings, we find very strong genetic correlation between anxiety (GAD-2) and psychiatric traits such as depression (r
g=0.81, p=2.48×10
−53) and neuroticism (r
g=0.72, p=7.09×10
−53) but relatively weaker genetic overlap with neurological disorders. Significant positive genetic association is detected for amyotrophic lateral sclerosis (r
g=0.39, p=3.00×10
−4), and negative genetic association with Parkinson’s disease (r
g=−0.19, p=4.70×10
−3), but no genetic overlap is seen for Alzheimer’s disease (r
g=0.00, p=1.00). Further work will be needed to better discern the implications of these findings for understanding shared and disparate disease mechanisms among these neuropsychiatric conditions (
11,
35,
51).
Limitations of this work include the fact that phenotypes were based on self-reported survey data. The GAD-2 asks questions that temporally reference the “past 2 weeks.” Although the GAD-2 has demonstrated high sensitivity and specificity for anxiety disorders (
52), it falls short of the desired trait (lifetime) anxiety measure. That our work reproduces (and is reproduced by) other independent groups who did use lifetime anxiety measures (
8,
9) further supports the utility of the GAD-2 in capturing a genetically meaningful anxiety trait. Similarly, the question about diagnosis for anxiety or panic that yielded our binary self-reported physician diagnosis of anxiety or panic disorder phenotype relies on self-report. A further limitation is that MVP has predominantly male participants (92.5%). While women are included in this analysis, clinically important interactions between sex, phenotype, and genotype could not be addressed. This cohort is growing, and future recruitment will provide additional power to revisit sex-stratified analyses of this sample. Males presumably have a higher genetic liability threshold for anxiety, as evidenced by lower rates of anxiety disorders. Accordingly, affected males could reflect higher genetic risk than females (because they must pass a higher threshold to be affected), which would result in greater power to detect risk loci in a mostly male compared with a mostly female sample.
In summary, we have identified novel variants for anxiety by performing GWASs in the large MVP cohort. We replicated results in our GWASs for top findings from recent anxiety and other relevant anxiety-related GWASs. We also identified significant genetic overlap with major depressive disorder, PTSD, and neuroticism using polygenic risk scores and LD score regression. This work provides additional genetic evidence for the overlap between disorders that are frequently comorbid with anxiety and presents new molecular targets for investigation with a longer view toward the development of new treatments.