Antipsychotic medications are essentially dopamine receptor antagonists, which may exert their effects via changes in brain circuitry. Therefore, neurobiological measures evaluating the function of brain circuitry would theoretically be potential candidates for such predictors. In line with this hypothesis, previous studies have shown that baseline brain activity and connectivity during certain functional MRI (fMRI) paradigms may relate to patients’ response status after antipsychotic treatment. Specifically, using resting-state fMRI, our own work has revealed that individualized calculators of striatal-cortical connectivity (
4) and cerebellar-cortical connectivity (
5) may be potential predictors for treatment outcome in first-episode psychosis. Similar findings have been reported in studies from independent laboratories with different treatment profiles (
6–
8), suggesting relative robustness of these results. In addition, functional connectivity measures regarding the default mode network areas (
9,
10), frontoparietal regions (
10), and sensorimotor cortices (
11,
12) were also found to be significantly correlated with symptom changes after medication, suggesting their potential clinical value as prognostic biomarkers. Beyond resting-state measures, task-based fMRI has also been demonstrated to be a useful tool for investigating potential predictors in patients. For instance, a recent study used a cognitive task to evaluate the function of performance monitoring in psychotic patients receiving treatment (
13). The investigators found that task-related activity in the frontoparietal regions at baseline significantly predicted the degree of symptom reduction after 1-year follow-up, which provides initial evidence for task-based imaging predictors. Nevertheless, most studies to date have used a hypothesis-driven approach that focuses on selected regions of interest with a specific fMRI paradigm. Since dopamine receptors are widely distributed across the whole brain and dopamine functions are widely involved in a variety of human behaviors (
14–
16), it is unlikely that neural predictors are located in highly circumscribed brain areas that can be detected during only one functional state. However, whether any trait-like functional biomarkers at the whole-brain connectome level would predict treatment outcome with antipsychotics is unknown.
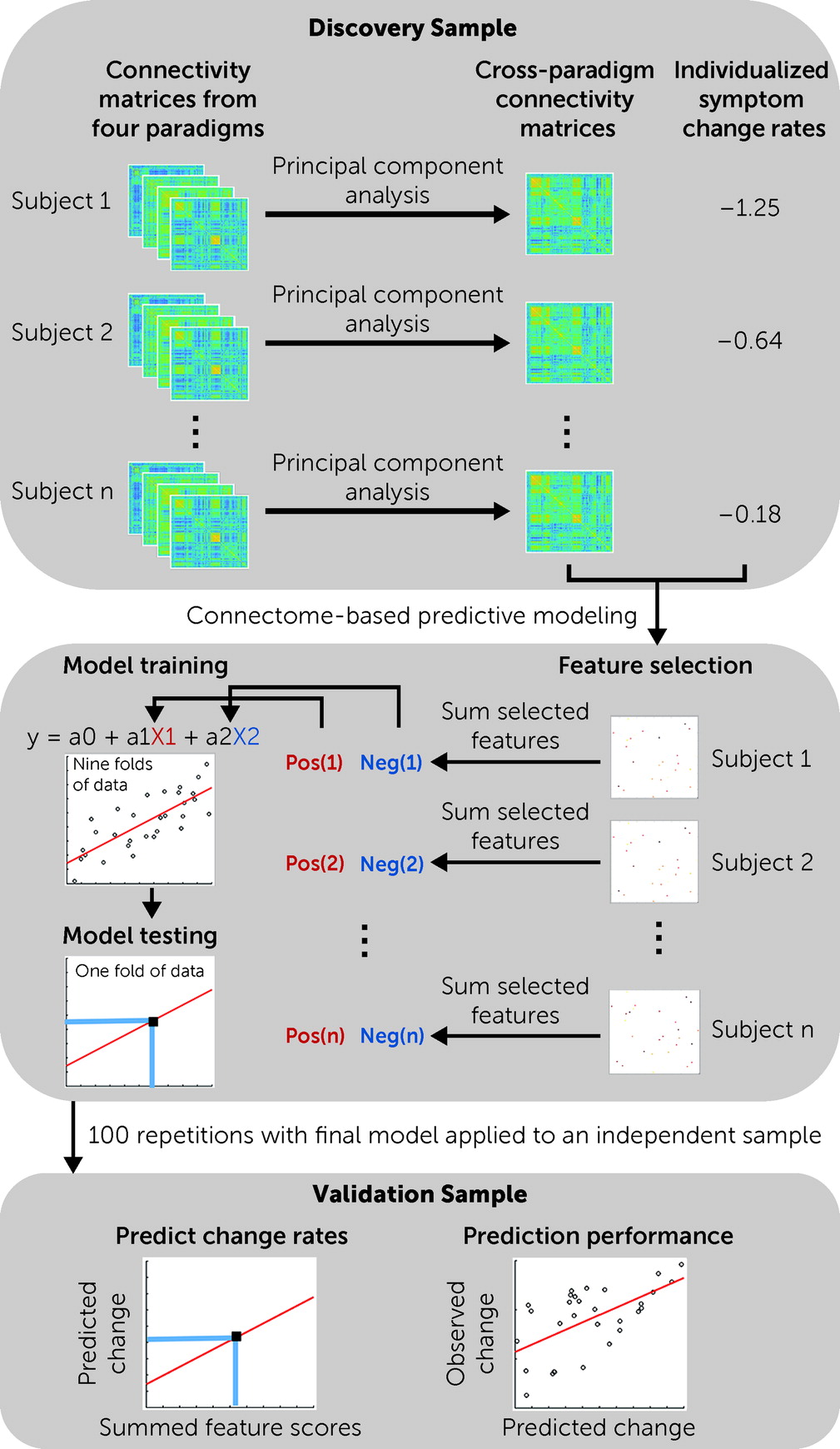
We previously proposed “cross-paradigm connectivity” (CPC) (
17) as a valid approach to evaluate “trait” organizations of the individual functional connectomes, which essentially extracts shared components across functional connectivity matrices constructed from different imaging paradigms (for a review, see reference
18). Compared with connectome measures constructed from a single paradigm (or “state”), the CPC matrices quantify individualized connectomic traits independent of brain functional state and show significantly higher test-retest reliability and individual predictability (
17). Implementing this approach in psychosis studies, we have successfully identified state-independent biomarkers for the prediction of illness onset among individuals at clinical high risk (
19) and state-independent neural mechanisms underlying schizophrenia polygenic risk (
20), implying its feasibility and utility in search of predictive biomarkers. Moreover, these past findings also suggest that psychotic disorders are associated with trait-like abnormalities at the connectome level, which encourages the exploration of such signatures for treatment prediction.
Discussion
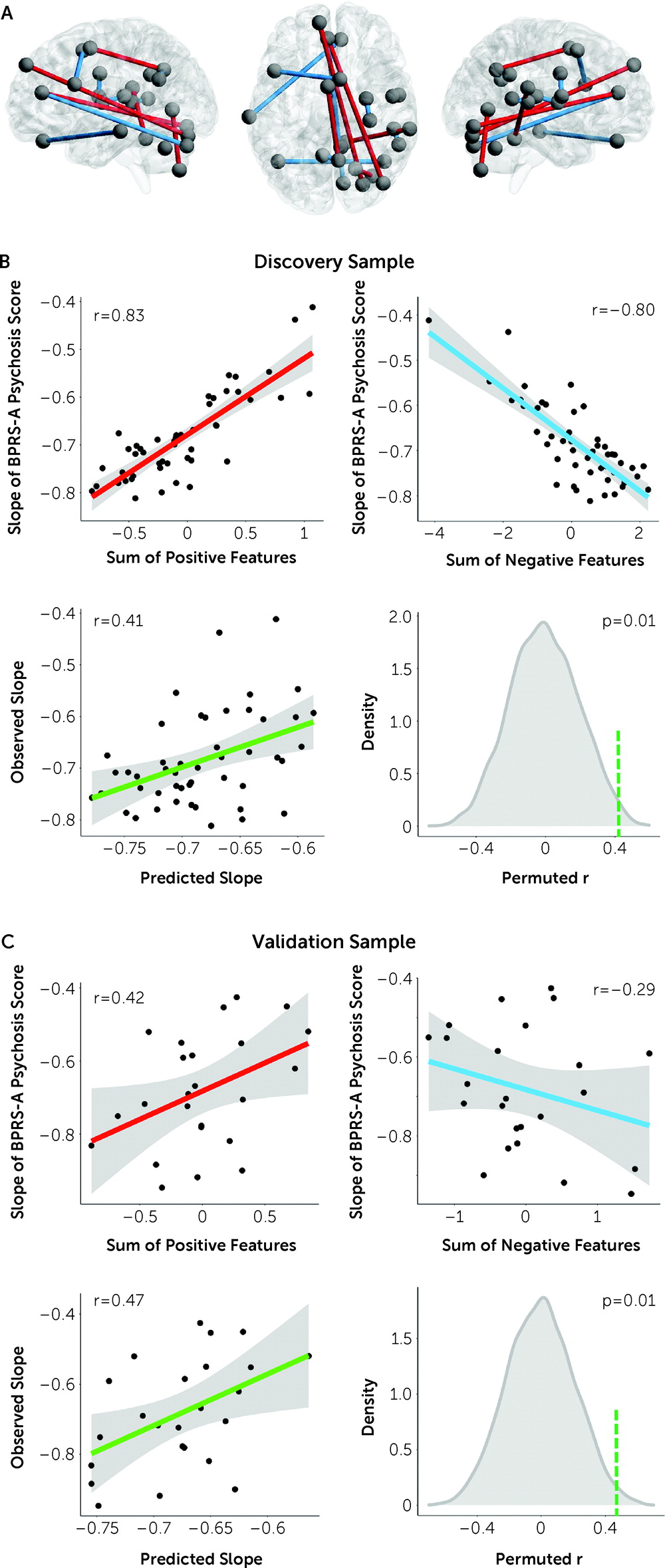
By combining cross-paradigm connectivity and connectome-based predictive modeling in a clinical sample, we identified a functional connectome-based neural signature for the prediction of individualized treatment outcome in patients with first-episode psychosis. This signature consisted of positive predictors predominantly related to the cerebellar-cortical circuitry and negative predictors chiefly within the cognitive systems in the cerebral cortex. We further demonstrated good generalizability of these predictors in an independent data set. Together, these findings highlight a promising trait-like functional biomarker that has potential to be leveraged as a prognostic predictor of treatment outcome in precision psychiatry.
While an increasing number of studies in the literature have aimed to investigate functional imaging predictors for treatment outcome in psychosis (
4–
12,
25,
26), they have so far focused on a single fMRI paradigm. Therefore, findings reported from past studies may reflect, at least in part, a state-related measure, making it difficult to distinguish whether the discovered biomarkers are related to an individualized trait that is predictive of treatment or a state-dependent change evoked by a specific paradigm that covaries with medication (
18). Our study overcame this limitation by using a completely data-driven approach combining multiple fMRI paradigms to identify neural traits most predictive of treatment response across the whole brain connectome regardless of the brain’s functional state. Our findings thus may point to a rudimentary neural mechanism tuning individualized responses to antipsychotic therapies in patients. One notable advantage of such an approach is that the findings would by nature facilitate clinical translation because they are not affected by experiment- or state-related factors and are generalizable between fMRI paradigms and data sets. As a proof of concept, our results indeed showed good generalizability across samples with different scan protocols, imaging paradigms, and preprocessing pipelines, suggesting that the detected predictors are robust and may show relatively high clinical value.
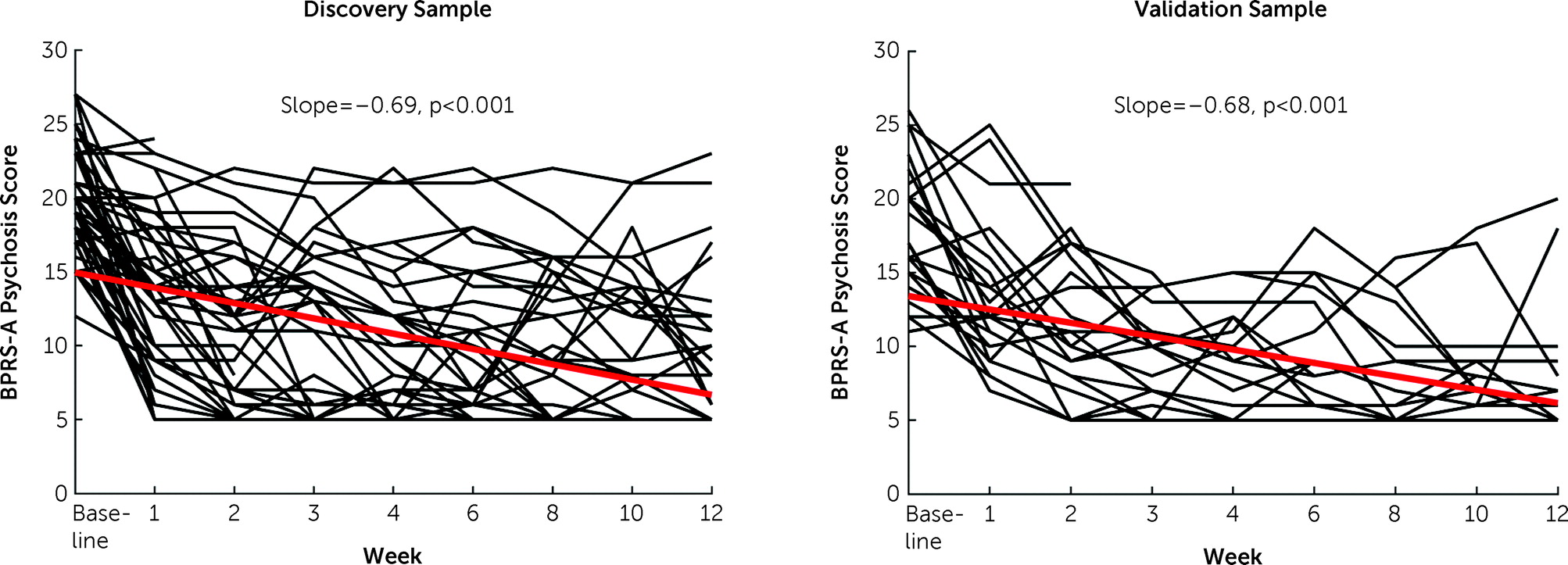
It is noteworthy that treatment prediction in this study was conducted at the individual level, which is different from the majority of studies in the literature dichotomizing patients into responders and nonresponders. Clearly, the degree of individualized response to antipsychotic drugs is a continuous variable, and binarized classification of treatment response is not only arbitrary but also statistically inefficient (
27,
28). In addition, such dichotomization completely ignores individual variabilities within each assigned group, as response heterogeneities are still evident among patients, even when they are uniformly assigned to a “responder” or “nonresponder” group. Our research therefore has the advantage of predicting the degree of symptom reduction for each individual. According to the mean squared error in the validation sample, it can be estimated that the mean deviation between the predicted and observed psychosis scores at week 12 is rather small (∼1.6) at the individual level, suggesting its potential to assist clinical judgment for individual patients.
The predictors identified here are broadly consistent with the psychosis literature, encompassing nodes in several key sensory processing systems (sensorimotor, auditory, visual) and higher-order cognitive systems (default mode, cingulo-opercular, frontoparietal, salience) that have repeatedly been reported in previous studies (
4–
12). Notably, two recent review articles suggested that connectivity in the default mode network and sensory networks were the most consistent predictors of treatment response (
29,
30). This conclusion generally aligns with the present results, as nodes in these systems were among those most frequently observed (see Table S1 in the
online supplement). Features negatively predictive of response were predominantly connections within the cognitive systems, in particular the default mode network, where patients with higher connectivity had better treatment response. Tentatively, since dopamine plays a key role in maintaining the normal function of cognitive processing, which gates the information input and output in order to balance goal-directed signals and background noise (
31,
32), stronger connectivity may reflect a result of dopamine dysregulation in the cortical cognitive systems. In this case, stronger connectivity may imply higher dopamine sensitivity (
33), rendering those individuals more responsive to antipsychotic treatment. Alternatively, it may also reflect a better cognitive capacity in the brain that facilitates the function of dopamine antagonists in rectification of a psychosis state (
34). It should be noted that the identified nodes may not be sufficient to represent these predefined networks, and therefore more comprehensive research is needed to further illustrate the function of these networks in treatment response. In addition, these nodes may also reflect a spatial shift of the network organization, as previously identified in patients (
35), and therefore individual variation in the topology of these networks needs to be further studied.
Intriguingly, the positive predictors are predominantly connections between the cerebellum and the cerebral cortex, where lower connectivity at baseline predicts better response to treatment. This is remarkably consistent with our previous findings that increased connectivity in the cerebellar-cortical circuitry is a trait abnormality for psychotic disorders (
19,
24). Such change can be robustly detected across different brain functional states and disease stages (
5,
19,
24,
36) and is predictive of illness onset in individuals at clinical high risk (
19). Moreover, higher connectivity in the cerebellar-cortical circuitry also significantly predicts worse clinical outcome after 2 years of continuous antipsychotic medication (
5). These lines of evidence converge to show that cerebellar-cortical hyperconnectivity is a highly robust pathological finding in psychosis, which has potential to be clinically utilized as a predictor of illness development and prognosis. While speculative, such abnormality has been suggested as a downstream effect of
N-methyl-
d-aspartate (NMDA) receptor hypofunction (
37,
38), which leads to the overactivity of pyramidal glutamatergic neurons and in turn disrupts the error processing signals conveyed between the cerebral cortex and the cerebellum (
39), thereby generating a series of aberrant thoughts and behaviors collectively theorized as “cognitive dysmetria” (
40,
41). Since the function of dopamine neurons is downstream modulated by glutamate signaling (
42–
44), it is plausible that such a trait would also affect antipsychotic treatment outcome in patients.
Beyond the glutamate hypothesis, it has increasingly been recognized that the bidirectional interactions between the cerebellum and the midbrain is a key and indispensable regulator of the brain’s dopaminergic system. In particular, it has been shown that the direct projection from the cerebellum to the midbrain powerfully modulates the firing rate of midbrain dopaminergic neurons and serves as a required mechanism for activating the reward circuitry and further contributing to social and cognitive behaviors (
45,
46). In addition, enriched dopamine D
2 receptors on the cerebellar Purkinje cells directly receive dopaminergic input from the midbrain, whose dysfunction has been shown to be sufficient to alter social functioning (
46,
47). As a result, cerebellar connectivity may be a pivotal factor in regulating dopamine function and in turn the antipsychotic effect on the brain. It remains to be determined, however, whether the functional modulation of cerebellar-cortical circuitry would boost individualized capacity for antipsychotic response.
We note that this study has some limitations. First, our study design did not include a placebo group, and therefore it is difficult to determine whether the detected predictors would relate to the placebo effect. This limitation may be common to psychosis medication studies in the literature, given that the inclusion of a placebo group is ethically difficult. Second, while we observed and validated the findings in two independent clinical samples, both samples were relatively small. Therefore, the generalizability of the results still merits further investigation in larger cohorts. For the same reason, we did not train predictive models for each drug (risperidone and aripiprazole) separately, and the reported findings may reflect a mixed effect of both drugs. However, in an exploratory analysis shown in the online supplement, we found that the predictive model applied to both drugs, suggesting potentially similar effects underlying the studied drugs. Third, our treatment response measure was primarily based on positive symptoms, which is the major target of antipsychotic drugs. Outcomes of other symptom domains, such as negative symptoms and social functioning, are also clinically important and should be investigated in the future. Fourth, while collecting multiple task–based fMRI data is demanding in clinical populations, further research is certainly warranted to optimize data acquisition strategy for better clinical translation. One possible solution, based on the state-independent nature of our findings, is to implement a similar approach in paradigms with much less cognitive demand, such as naturalistic stimuli. Finally, because of small sample sizes, we did not calculate slopes separately for the training and testing samples in the discovery data set, and therefore the estimated slopes may not be independent due to the partial pooling effect in the linear mixed model. However, since our findings were validated in an independent data set in which the slopes were calculated separately, the prediction performance is unlikely to be driven simply by data leakage.
In summary, using multi-paradigm fMRI data from two clinical samples, we discovered and validated a trait-like connectomic signature as individualized predictors of antipsychotic response in first-episode psychosis. The identified predictors highlight the potential roles of the cerebellar-cortical circuitry and cortical cognitive systems in prediction of psychosis outcome. Additional studies are encouraged to further test the translational value of this neural signature in clinical settings.