MDMA and MDMA-Assisted Therapy
Abstract
History of MDMA and MDMA-Assisted Therapy
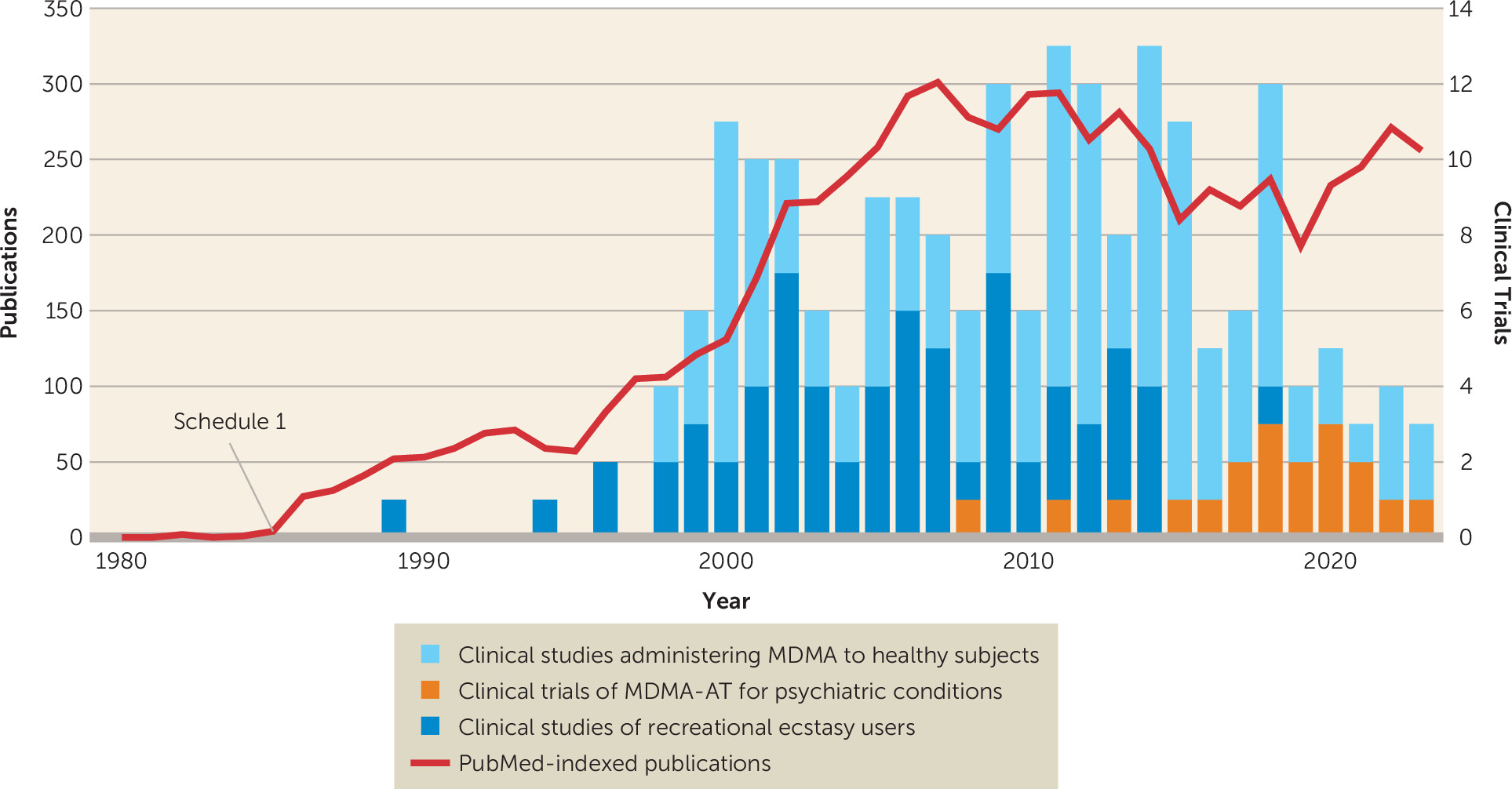
MDMA: What It Is and What It Is Not
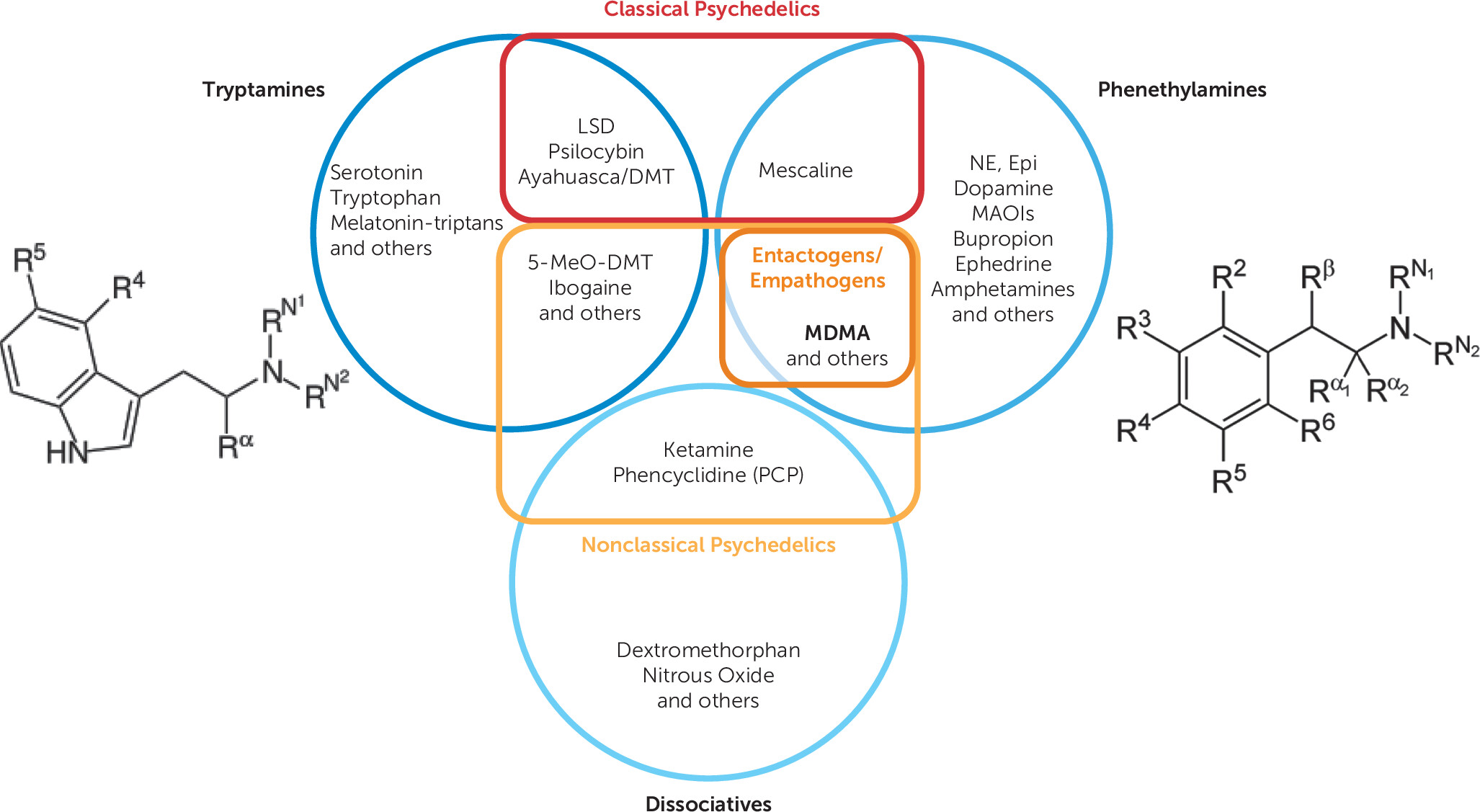
Classification and Subjective Effects
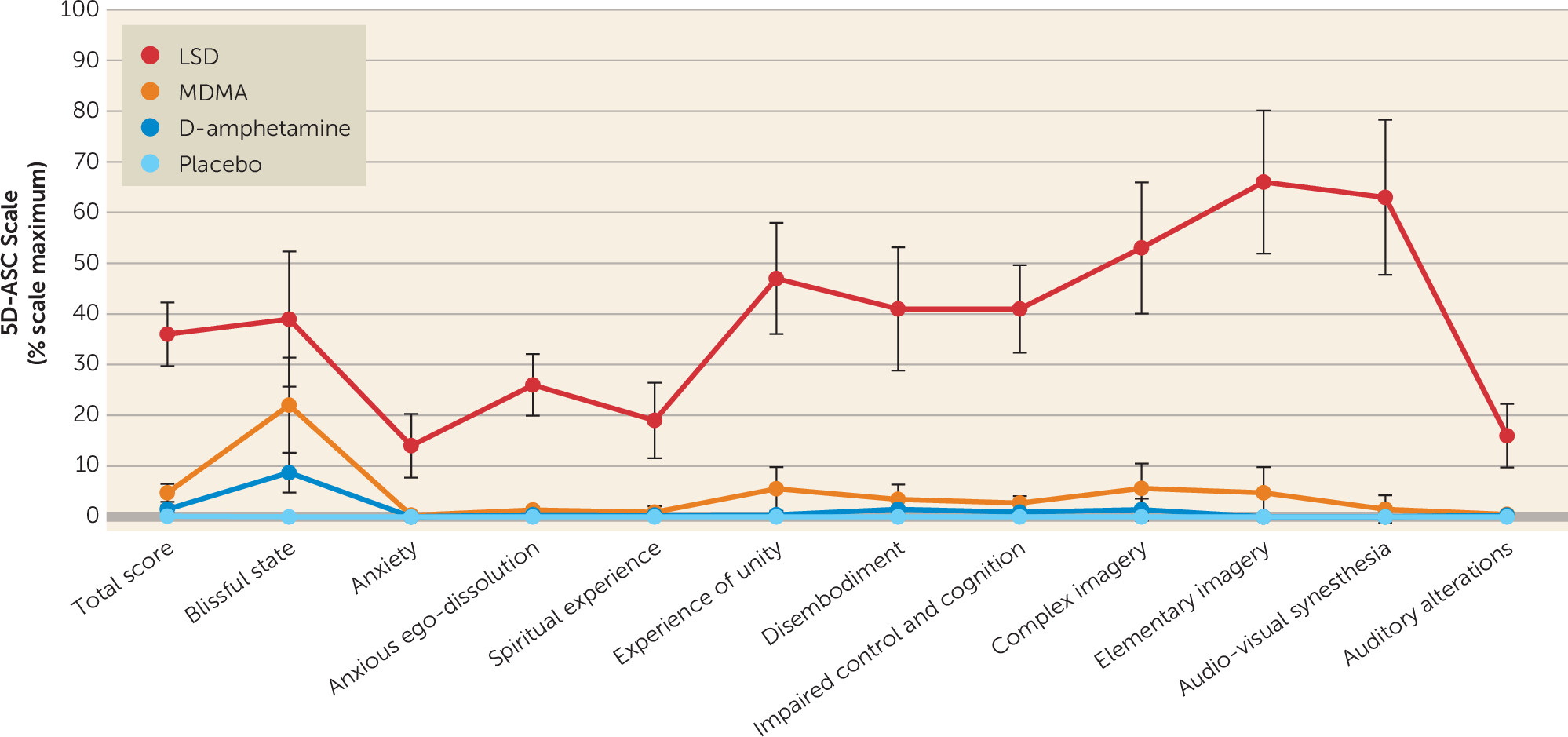
Objective Effects
Pharmacology and Neuroscience
Safety of MDMA and MDMA-Assisted Therapy
“Ecstasy” Versus MDMA
Neurotoxicity
Mortality
Hyperthermia
Hyponatremia
Cardiovascular Conditions
Psychiatric Conditions
Neurocognition
Addiction
Serotonin Syndrome
Relational Safety
Therapeutic touch.
Sexual abuse.
Interpersonal dependency.
Suggestibility.
Summary: Safety of MDMA and MDMA-AT
MDMA-Assisted Therapy
Treatment Course
Therapeutic Modality
MDMA-AT for PTSD
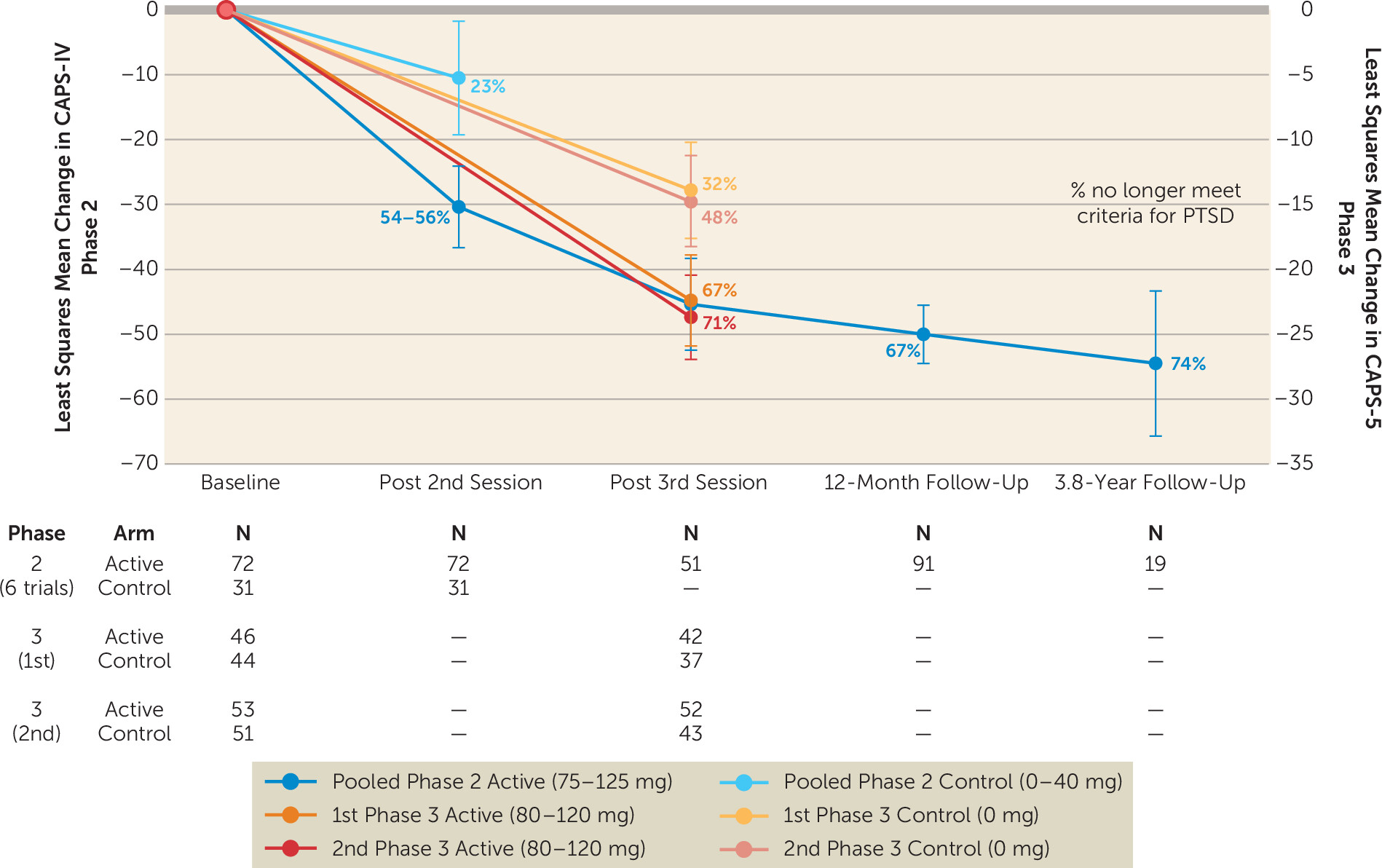
Efficacy.
| Study (FDA phase) | Study design | Dose (mg)b | Group size (N) | Veterans/Combat trauma (N) | Clinical response at primary endpoint | Loss of PTSD diagnosis at primary endpoint | Loss of PTSD diagnosis at 12 monthsc | CAPS-IV/5 Between-group effect size of primary outcome (Cohen’s d) |
|---|---|---|---|---|---|---|---|---|
| Mithoefer et al., 2011 (Phase 2) (106) | RCT; two closed-label MDMA sessions; open-label crossover of placebo arm for two more 125-mg sessions; primary outcome: CAPS-IV 2 months after second closed-label MDMA session. | 125 and 0 | 125 mg: 12 0 mg: 8 | 125 mg: 1 | 125 mg: 10/12 (83%) 0 mg: 2/8 (25%) | 125 mg: 10/12 (83%) 0 mg: 2/8 (25%) | All: 14/16 (88%)d | 1.24 |
| Oehen et al., 2013 (Phase 2) (108) | RCT; three closed-label MDMA sessions; open-label crossover of placebo arm for three more 125-mg sessions; primary outcome: CAPS-IV 3 weeks after third closed-label MDMA session. | 125 and 25 | 125 mg: 8 25 mg: 4 | None | 125 mg: 4/8 (50%) 25 mg: 0/4 (0%) | 125 mg: 0/8 (0%) 25 mg: 0/4 (0%) | All: 5/12 (42%) | |
| Mithoefer et al., 2018 (Phase 2) (86) | RCT; two closed-label MDMA sessions; 125-mg arm did one more open-label session; 75-mg and 30-mg arms crossed over and did three more 100–125-mg open-label sessions; primary outcome: CAPS-IV 1 month after second closed-label MDMA session. | 125, 75, and 30 | 125 mg: 12 75 mg: 7 30 mg: 7 | 125 mg: 9 75 mg: 7 30 mg: 6 | 125 mg: 8/12 (67%) 75 mg: 7/7 (100%) 30 mg: 2/7 (29%) | 125 mg: 7/12 (58%) 75 mg: 6/7 (86%) 30 mg: 2/7 (29%) | 125 mg: 8/11 (72%) 75 mg: 5/7 (71%) 30 mg: 3/6 (50%) | 1.1e, 2.8f |
| Ot’alora et al., 2018 (Phase 2) (109) | RCT; two closed-label MDMA sessions; 125-mg and 100-mg arms did one more open-label session; 40-mg arm crossed over and did three more 100–125 mg open-label sessions; primary outcome: CAPS-IV 1 month after second closed-label MDMA session. | 125, 100, and 40 | 125 mg: 13 100 mg: 9 40 mg: 6 | g | 125 mg: 6/12 (50%) 100 mg: 5/9 (56%) 40 mg: 1/6 (17%) | 125 mg: 5/12 (42%) 100 mg: 4/9 (44%) 40 mg: 2/6 (33%) | All: 19/25 (76%) | 1.12, 0.73h |
| Mitchell et al., 2021 (Phase 3) (2) | RCT; three closed-label MDMA sessions, no open-label cross-over; primary outcome: CAPS-5 1 month after third closed-label MDMA session. | 80–120 and 0i | 80–120 mg: 46 0 mg: 44 | 80–120 mg: 10 0 mg: 6 | j | 80–120 mg: 28/42 (67%) 0 mg: 12/37 (32%) | 0.91 | |
| Mitchell et al., 2023 (Phase 3) (3) | RCT; three closed-label MDMA sessions, no open-label cross-over; primary outcome: CAPS-5 1 month after third closed-label MDMA session. | 80–120 and 0i | 80–120 mg: 53 0 mg: 51 | 80–120 mg: 9 0 mg: 7 | 80–120 mg: 45/52 (87%) 0 mg: 29/42 (69%) | 80–120 mg: 37/52 (71%) 0 mg: 20/42 (48%) | 0.70 |
Comparisons to current PTSD treatments.
Comorbid depression and insomnia.
Posttraumatic growth.
Personality.
Other studies of MDMA-AT for PTSD.
MDMA-AT for Other Conditions
Alcohol use disorder.
Anxiety.
Tinnitus.
Limitations
Future Directions
Pharmacology: MDMA Derivatives and Analogs
Investigating Therapeutic Mechanisms
Examining and Developing the Therapeutic Modality
Novel Indications
| Study and treatment arm | Condition | Study design | Dose (mg) | Group size | Notes |
|---|---|---|---|---|---|
| Bouso et al., 2008 (104) | PTSD | RCT; one closed-label MDMA session | 75 and 50 | 75 mg (N=1); 50 mg (N=3); placebo (N=2) | First clinical trial of MDMA-AT; conducted in Spain; pilot study planned for 29 participants but closed early due to political pressures |
| Pacey et al., unpublished (119) | PTSD | RCT; two closed-label MDMA sessions; active arm did one more open-label session; placebo arm did three more open label MDMA sessions | 125 and 25 | 125 mg (N=4); 25 mg (N=2) | Data unpublished in the peer reviewed literature although are included in the secondary analyses and can be found on clinicaltrials.gov |
| Kotler et al., unpublished (154) | PTSD | RCT; two closed-label MDMA sessions; placebo arm did two more open-label MDMA sessions | 125 and 25 | 125 mg (N=5); 25 mg (N=3) | Data unpublished in the peer reviewed literature although are included in the secondary analyses and can be found on clinicaltrials.gov |
| Monson et al., 2020 (107) | PTSD | Two open-label, single-arm MDMA sessions; CBCT protocol, both patient and spouse administered together | 125 | N=6b | Pilot study; of the six individuals five (83%) no longer diagnosable with PTSD; outcomes of relationship quality and satisfaction also improved. |
| Jardim et al., 2021 (179) | PTSD | Three open-label, single-arm MDMA sessions | 125 | N=3 | Pilot study; all three patients had clinically significant improvements in CAPS-IV scores. |
| Danforth et al., 2018 (105) | Social anxiety in adults with autism spectrum disorder | RCT; two closed-label MDMA sessions | 75–125 and 30 | 75-125 mg (N=8); 30 mg (N=4) | Social anxiety as measured by LSAS remitted in 71% (N=5 of 7) of MDMA group versus 50% (N=2) of placebo group; effects were large (Cohen’s d=1.4) and durable at 6 months |
| Wolfson et al., 2020 (111) | Anxiety related to life-threatening illness | RCT; two closed-label MDMA sessions; active group received one more open-label MDMA session; placebo group received three open-label MDMA sessions | 125 | MDMA (N=13); placebo (N=5) | Large effect size in reduction of anxiety (Hedge’s g=1.03), although not statistically significant (p=0.056). |
| Sessa et al., 2021 (192) | Alcohol use disorder | Two open-label, single-arm MDMA sessions after alcohol detoxification; post-detoxification treatment-as-usual observational study used as comparator | 125 | N=14 | 100% of participants drank >14 units of alcohol per week at baseline compared with 14% at 2–3 months—and 21% at 9 months—after detoxification and completing a course of MDMA-AT; observational study showed 75% drank >14 units of alcohol 9 months after detoxification |
| Searchfield et al., 2020 (180) | Tinnitus | Randomized crossover trial; two-part study: 1) crossover of 30 mg and placebo, and 2) crossover of 70 mg and placebo | 70 and 30 | 70 mg (N=8); 30 mg (N=5) | Pilot study; no significant between-group changes in primary or long-term outcomes; significant changes in secondary outcomes of annoyance and ability to ignore ringing |
Care Delivery Models and Resource Intensiveness
Public Policy and Access for Nonmedical Use
Research Funding Support
Conclusions
Supplementary Material
- View/Download
- 184.04 KB
References
Information & Authors
Information
Published In
History
Keywords
Authors
Competing Interests
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

