The Treatment of Restless Legs Syndrome and Periodic Limb Movement Disorder in Adults—An Update for 2012: Practice Parameters with an Evidence-Based Systematic Review and Meta-Analyses: An American Academy of Sleep Medicine Clinical Practice Guideline
Abstract
1.0 Introduction
2.0 Background
2.1 Diagnosis
2.2 Treatment Efficacy Measures
3.0 Methods
3.1 Literature Search
3.2 PICO Questions
| Population | Intervention | Comparison | Outcome |
|---|---|---|---|
| Adults diagnosed with RLS using the ICSD-2 or the International RLS Study Group (IRLS) diagnostic criteria | Pramipexole | Control group, those with untreated RLS, or those with RLS using an alternate treatment | Subjective measures: |
| Ropinirole | 1. IRLS rating scale | ||
| Levodopa | 2. Clinical Global Impression (CGI) Scale | ||
| Pergolide | 3. RLS-6 | ||
| Cabergoline | 4. Patient Global Impression (PGI) | ||
| Opioids | 5. Sleep Questionnaire Form A | ||
| Gabapentin Enacarbil | 6. Quality of Life (QoL) for RLS | ||
| Gabapentin | 7. Augmentation Severity Rating Scale (ASRS) | ||
| Pregabalin | 8. Visual Analog Scales (VAS) | ||
| Carbamazepine | 9. Medical Outcomes Study Sleep Scale (MOS) | ||
| Clonidine | 10. Self-Rating Zung Depression Scale (SDS) | ||
| Iron supplementation | 11. Anxiety Scale (SAS) | ||
| Rotigotine | 12. SF-36 | ||
| Lisuride | 13. Work productivity and activity impairment (WPAI) | ||
| Amantadine | 14. Pittsburgh Sleep Quality Index (PSQI) | ||
| Talipexole | 15. Hospital Anxiety and Depression Scale (HADS) | ||
| Peribedil | 16. Subjective sleep and awakening quality scale (SSA) | ||
| Alpha-dihydroergocryptine | 17. Epworth Sleepiness Scale (ESS) | ||
| Clonazepam | Objective measures: | ||
| Valproic acid | 1. Sleep-related parameters by polysomnography | ||
| Valerian | a. PLMS | ||
| Antidepressants | b. PLMI | ||
| c. PLMS-AI | |||
| d. Sleep efficiency | |||
| e. TST | |||
| f. % TIB without leg movements | |||
| g. PLMWI | |||
| 2. Sleep-related parameters by actigraphy | |||
| a. Leg movements | |||
| b. Sleep efficiency |
3.3 Meta-Analysis
3.4 Quality of Evidence
| Study design | Initial quality of a body of evidence | Lower if | Higher if | Quality of a body of evidence |
|---|---|---|---|---|
| Radomized trials | High → | Risk of bias | Large effect | High (four plus:⊕⊕⊕⊕) |
| −1 Serious | +1 Large | |||
| −2 Very serious | +2 Very large | |||
| Inconsistency | Dose response | Moderate (three plus:⊕⊕⊕○) | ||
| −1 Serious | +1 Evidence of a gradient | |||
| −2 Very serious | All plausible residual confounding | |||
| Observational studies | Low → | Indirectness | +1 Would reduce a demonstrated effect | Low (two plus:⊕⊕○○) |
| −1 Serious | +1 Would suggest a spurious effect if no effect was observed | |||
| −2 Very serious | ||||
| Imprecision | Very Low (one plus:⊕○○○) | |||
| −1 Serious | ||||
| −2 Very serious | ||||
| Publication bias | ||||
| −1 Likely | ||||
| −2 Very likely |
Box 1—Final assessments of level of bodies of evidence1
3.5 Strength of Recommendations
| Overall quality of evidence | |||||
|---|---|---|---|---|---|
| High | Moderate | Low | Very Low | ||
| Assessment of benefit/harm/burden | Benefits clearly outweigh harm/burden | Standard | Standard | Guideline | Option |
| Benefits closely balanced with harm/burden OR uncertainty in the estimates of benefit/harm/burden | Guideline | Guideline | Option | Option | |
| Harm/burden clearly outweighs benefits | Standard | Standard | Standard | Standard | |
4.0 Recommendations for Therapies for RLS
| Practice Parameter | Strength of Recommendation | Body of Evidence Level | Harm/burden Assessment | FDA status |
|---|---|---|---|---|
| Standards for use in RLS | ||||
| Clinicians should treat patients with RLS with pramipexole. | (STANDARD) | High | Benefits clearly outweigh harms | Approved for indication |
| Clinicians should treat patients with RLS with ropinirole. | (STANDARD) | High | Benefits clearly outweigh harms | Approved for indication |
| Standards against use in RLS | ||||
| Clinicians should not treat RLS patients with pergolide because of the risks of heart valve damage. | (STANDARD) | High | Harms clearly outweigh benefits | Discontinued |
| Guidelines for use in RLS | ||||
| Clinicians can treat RLS patients with levodopa with dopa decarboxylase inhibitor. | (GUIDELINE) | High | Benefits closely balanced with harms. This is particularly true for those with intermittent RLS who use this medication sporadically. | Approved, but off-label use |
| Clinicians can treat RLS patients with opioids. | (GUIDELINE) | Low | Benefits clearly outweigh harms | Approved, but off-label use |
| Clinicians can treat patients with RLS with gabapentin enacarbil. | (GUIDELINE) | High | Uncertainty in balance between benefits and harms | Approved for indication |
| Given the potential of side effects, including heart valve damage, clinicians can treat RLS patients with cabergoline only if other recommended agents have been tried first and failed, and close clinical follow-up is provided. | (GUIDELINE) | High | Benefits closely balanced with harms | Approved, but off-label use |
| Options for use in RLS | ||||
| Clinicians may treat RLS patients with gabapentin. | (OPTION) | Low | Unclear benefit/harm balance | Approved, but off-label use |
| Clinicians may treat patients with RLS with pregabalin. | (OPTION) | Low | Benefits closely balanced with harms | Approved, but off-label use |
| Clinicians may treat RLS patients with carbamazepine. | (OPTION) | Low | Benefits closely balanced with harms | Approved, but off-label use |
| Clinicians may treat RLS patients with clonidine. | (OPTION) | Low | Unclear benefit/harm balance | Approved, but off-label use |
| Clinicians may use supplemental iron to treat RLS patients with low ferritin levels. | (OPTION) | Very Low | Unclear benefit/harm balance | Approved, but off-label use |
| PLMD | ||||
| There is insufficient evidence at present to evaluate the use of pharmacological therapy in patients diagnosed with PLMD alone. | (NO RECOMMENDATION) | Insufficient | N/A | N/A |
4.1 Introduction to Therapies for RLS
4.2 Pharmacotherapy
4.2.1 Dopaminergic medications
4.2.1.1 Non-ergot derived dopamine agonist: pramipexole
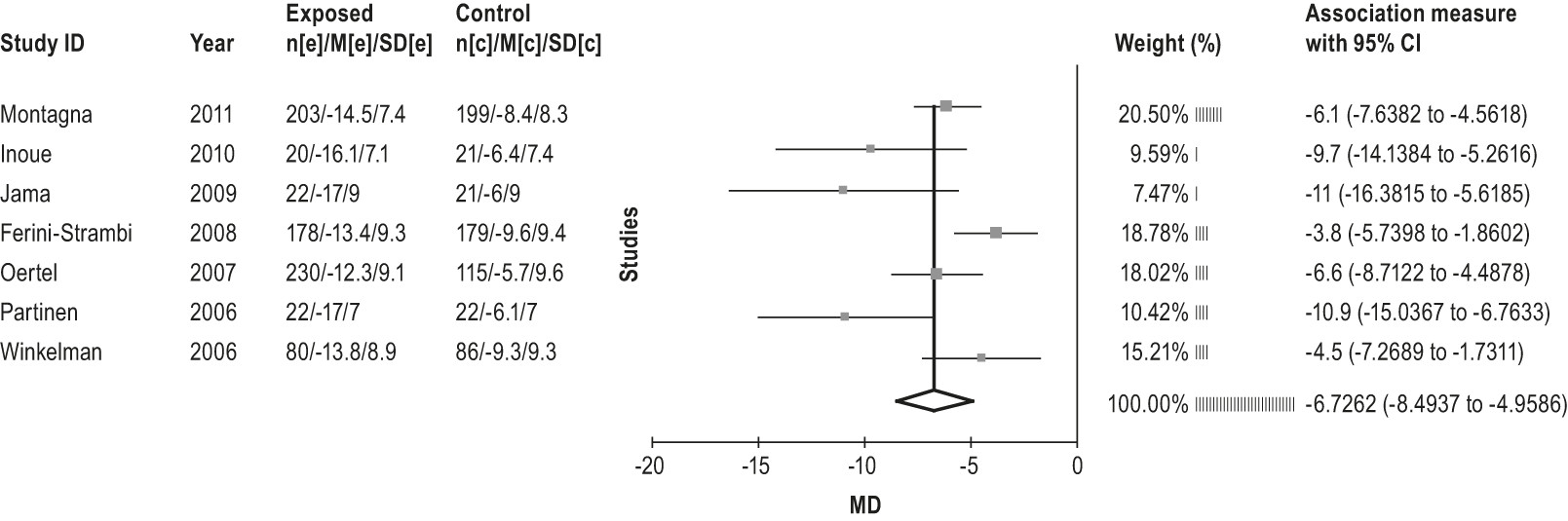
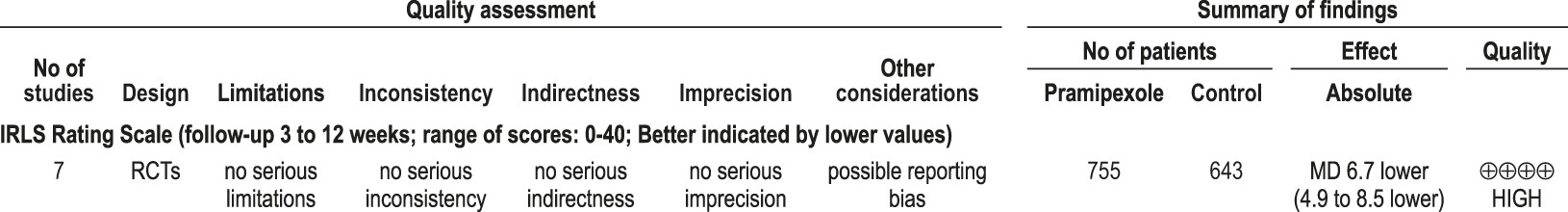
4.2.1.1a: Clinicians should treat patients with RLS with pramipexole. (STANDARD)
4.2.1.2 Non-ergot derived dopamine agonist: ropinirole
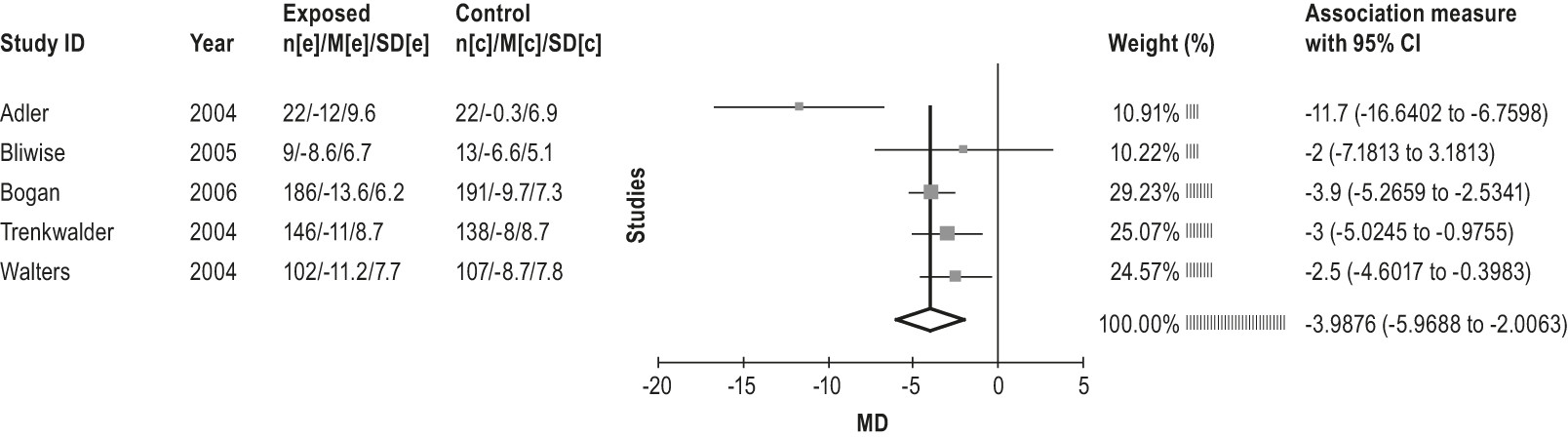
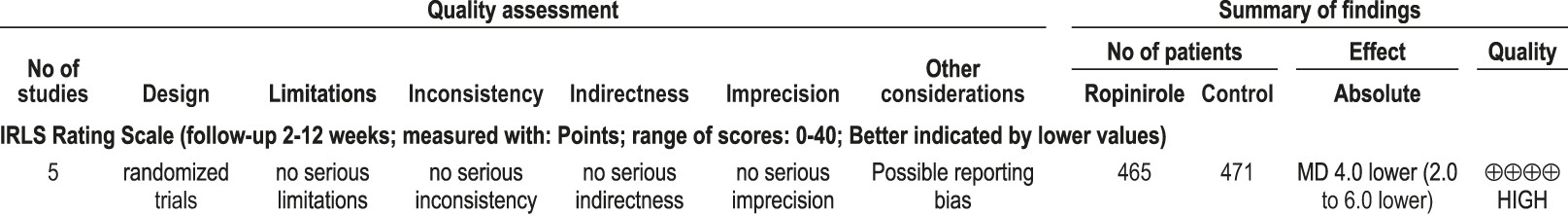
4.2.1.2a: Clinicians should treat patients with RLS with ropinirole. (STANDARD)
4.2.1.3 Levodopa
4.2.1.3a: Clinicians can treat RLS patients with levodopa with dopa decarboxylase inhibitor. (GUIDELINE)
4.2.1.4 Ergot-derived dopamine agonists: pergolide and cabergoline
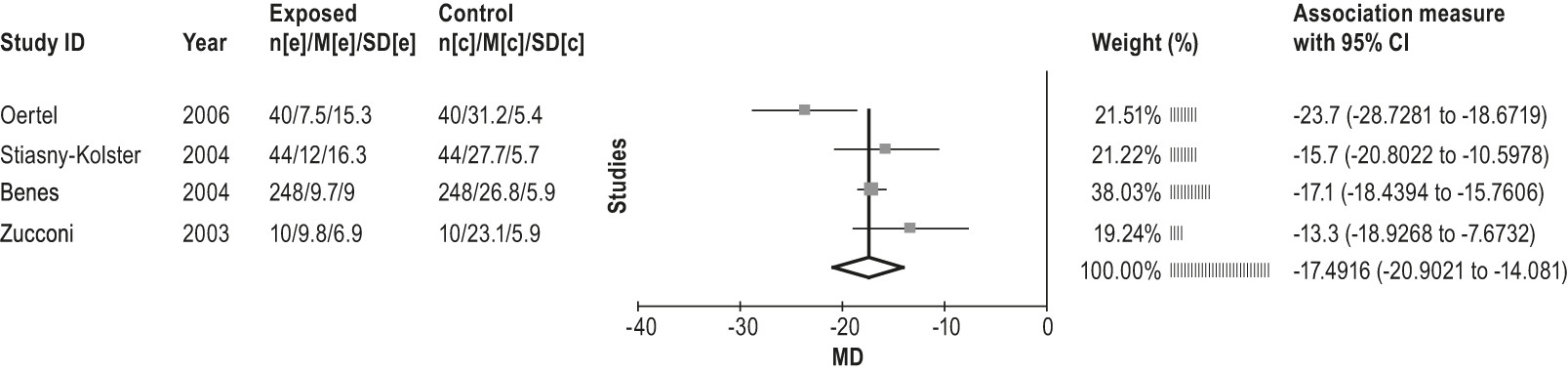
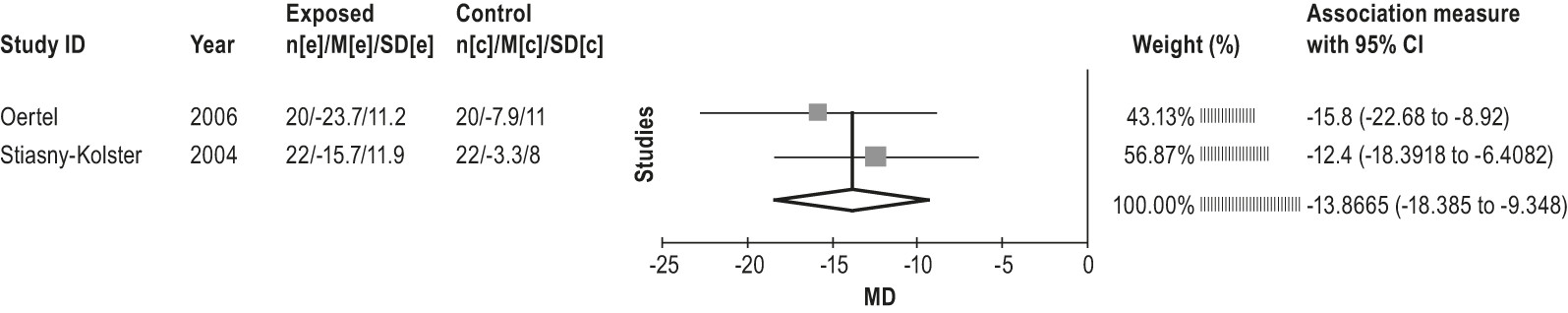
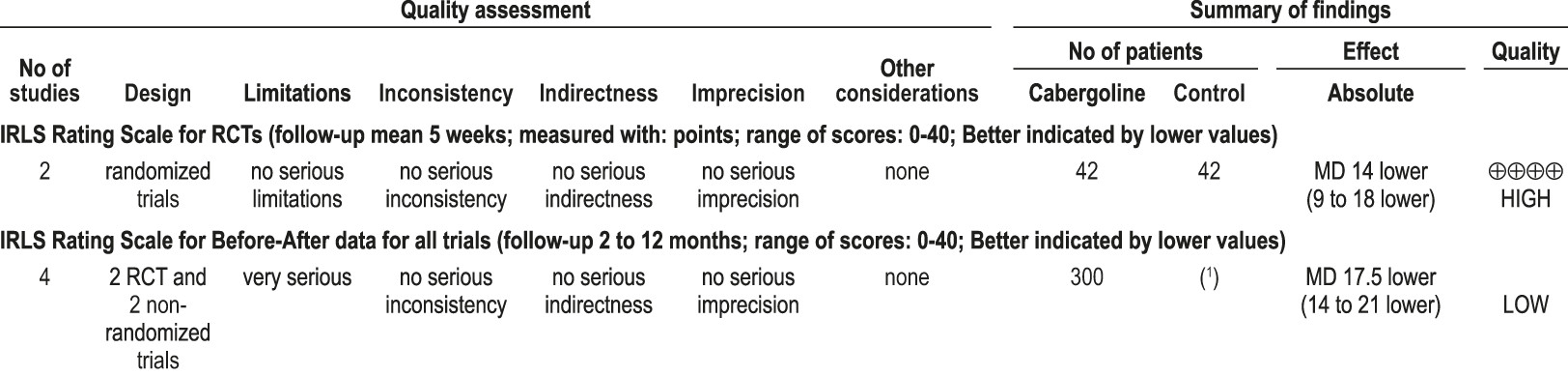
4.2.1.4a: Clinicians should not treat RLS patients with pergolide because of the risks of heart valve damage. (STANDARD)
4.2.1.4b: Given the potential of side effects, including heart valve damage, clinicians can treat RLS patients with cabergoline only if other recommended agents have been tried first and failed, and close clinical follow-up is provided. (GUIDELINE)
4.2.2 Opioid medications
4.2.2a: Clinicians can treat RLS patients with opioids. (GUIDELINE)
4.2.3 Anticonvulsant medications
4.2.3.1 Gabapentin enacarbil
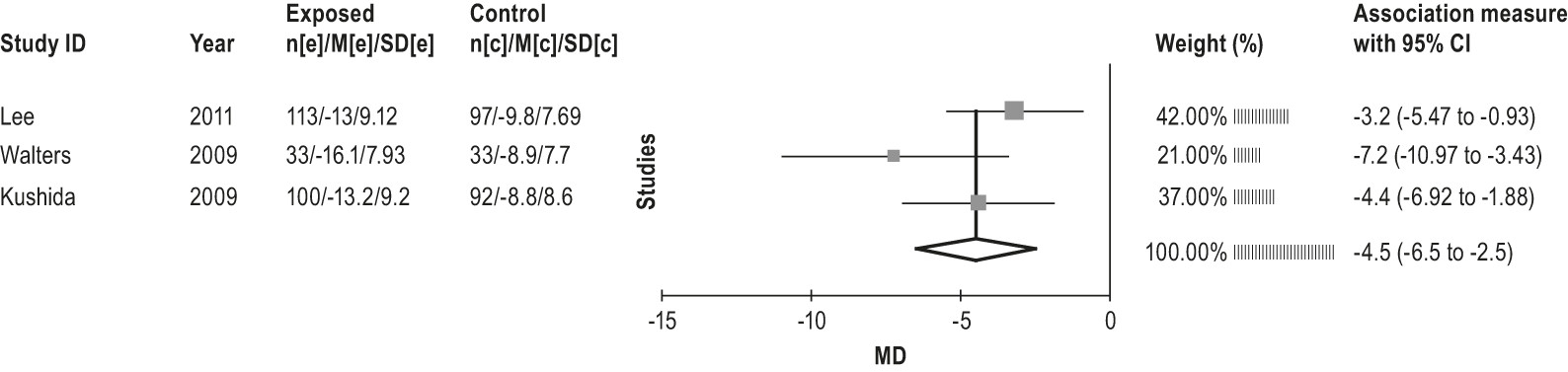

4.2.3.1a: Clinicians can treat patients with RLS with gabapentin enacarbil. (GUIDELINE)
4.2.3.2 Gabapentin

4.2.3.2a: Clinicians may treat RLS patients with gabapentin. (OPTION)
4.2.3.3 Pregabalin

4.2.3.3a: Clinicians may treat patients with RLS with pregabalin (OPTION)
4.2.3.4 Carbamazepine
4.2.3.4a: Clinicians may treat RLS patients with carbamazepine. (OPTION)
4.2.4 Medications acting on the adrenergic systems
4.2.4a: Clinicians may treat patients with RLS with clonidine (OPTION)
4.2.5 Iron supplementation
4.2.5a: Clinicians may use supplemental iron to treat RLS patients with low ferritin levels. (OPTION)
4.3 Therapies For Which No Recommendations Are Made
4.3.1 Non-ergot-derived dopamine agonists: rotigotine
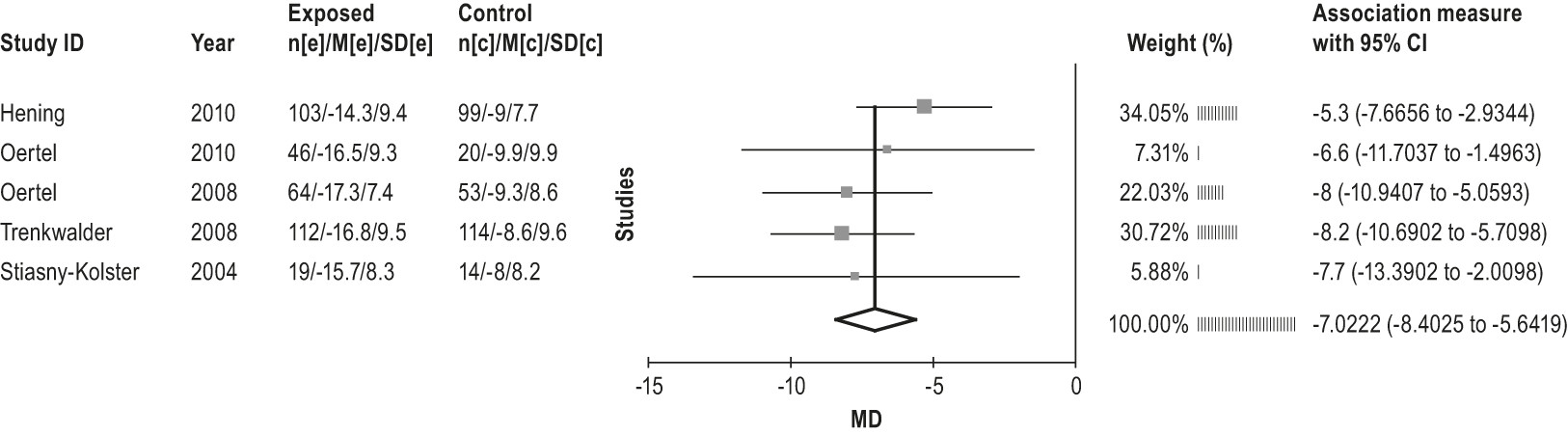
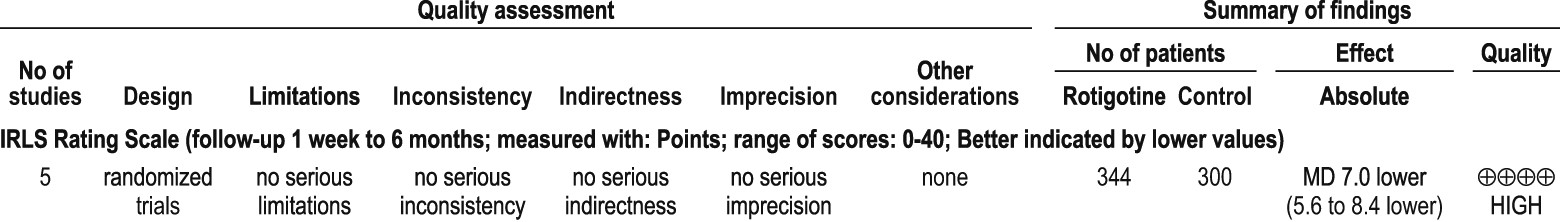
4.3.2 Other dopaminergic medications: lisuride and amantadine
4.3.3 Other dopamine agonists
4.3.4 Benzodiazepines (clonazepam)
4.3.5 Valproic acid
4.3.6 Valerian
4.3.7 Avoidance of antidepressants
4.3.8 Non-pharmacological therapy
4.3.9 Secondary RLS and special patient groups
5.0 Therapies for PLMD
5.1 Clonazepam
5.2 Melatonin
5.3 Valproate
5.4 Selegiline
5.0a: There is Insufficient Evidence at Present to Comment on the use of Pharmacological Therapy in Patients Diagnosed With PLMD Alone. (NO RECOMMENDATION)
6.0 Conclusions and Future Directions
Footnote
Supplementary Material
- View/Download
- 42.37 KB
References
Information & Authors
Information
Published In
History
Authors
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBGet Access
Login options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

