Human adaptive behavior requires neural systems that mediate the evaluation of stimuli in terms of the well-being of the organism and the subsequent generation of goal-directed behavior. In this article, we provide an overview of these systems, with an emphasis on those related to positive motivation/approach and review current knowledge on motivational processing and behavior, including relevant contributions from a variety of disciplines allied with the cognitive/affective neurosciences. We illustrate, with an emphasis on systems level function, how the various phenomena and terms encountered in clinical neuropsychiatry may be broadly viewed in light of this knowledge and discuss dysfunction of motivational systems in the setting of multiple neuropsychiatric disorders speaking broadly in terms of deficits, dysregulation, excess, and related syndromes. Illustrative examples are provided, with an emphasis on functional neuroimaging studies. This approach can provide a foundation for conceptualization, diagnosis, and targeted neuromodulatory therapeutics of neuropsychiatric disorders and is consistent with the National Institute of Mental Health (NIMH) Research Domain Criteria (RDoC) project, an initiative that seeks to develop new ways of classifying psychopathology based on dimensions of observable behavior and neurobiological measures (see
http://www.nimh.nih.gov/research-priorities/rdoc/index.shtml). The goal of the initiative is to define basic dimensions of functioning across multiple units of analysis, cutting across disorders as traditionally defined, to translate progress in basic neurobiological and behavioral research to an improved integrative understanding of psychopathology and the development of new and/or optimally matched treatments for mental disorders. The RDoC research framework currently consists of five major domains of functioning with related subconstructs, including a Positive Valence Systems domain with a subconstruct of Approach Motivation, centered on the mesolimbic dopamine pathway, which is the focus of this article. Approach motivation can in turn be divided into several components: wanting (related terms include motivation, anticipation, incentive salience, and appetitive behavior), liking (related terms include pleasure, hedonia, and consummatory behavior), and incentive learning or prediction, with each mediated by differing control systems within nucleus accumbens-ventral pallidal pathways and modulated by differing neurochemical signals, as discussed below.
Neural Systems Underlying Motivation/Emotion
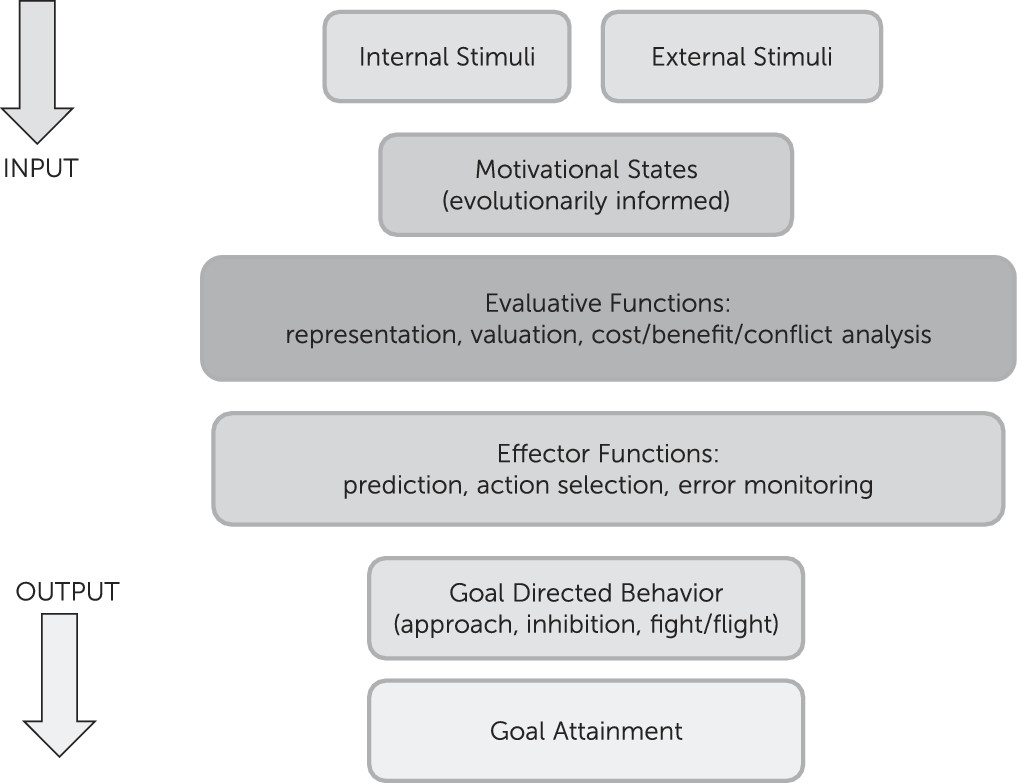
Motivation/emotion can be viewed as an adaptational system, comprised of evaluative and effector/behavioral arms that produces behaviors in response to changing internal and environmental conditions. This system has been shaped by natural selection to promote behaviors that increase the likelihood of genetic propagation via survival, sexual reproduction, and subsequent survival of offspring/relatives. Basic evaluation involves comparison of information, of external or internal origin, with stored information attained through experience or inheritance, to assess its significance for the evolutionary well-being of the organism. Basic behaviors include fight or flight from predators, inhibition of ongoing behavior in response to stimuli associated with punishment or omission of reward, and approach to positive/appetitive stimuli (
Figure 1). Those components of the system involved in evaluation and subsequent action related to positive stimuli are commonly termed reward processes and are discussed below within the context of the broader motivational/emotional system.
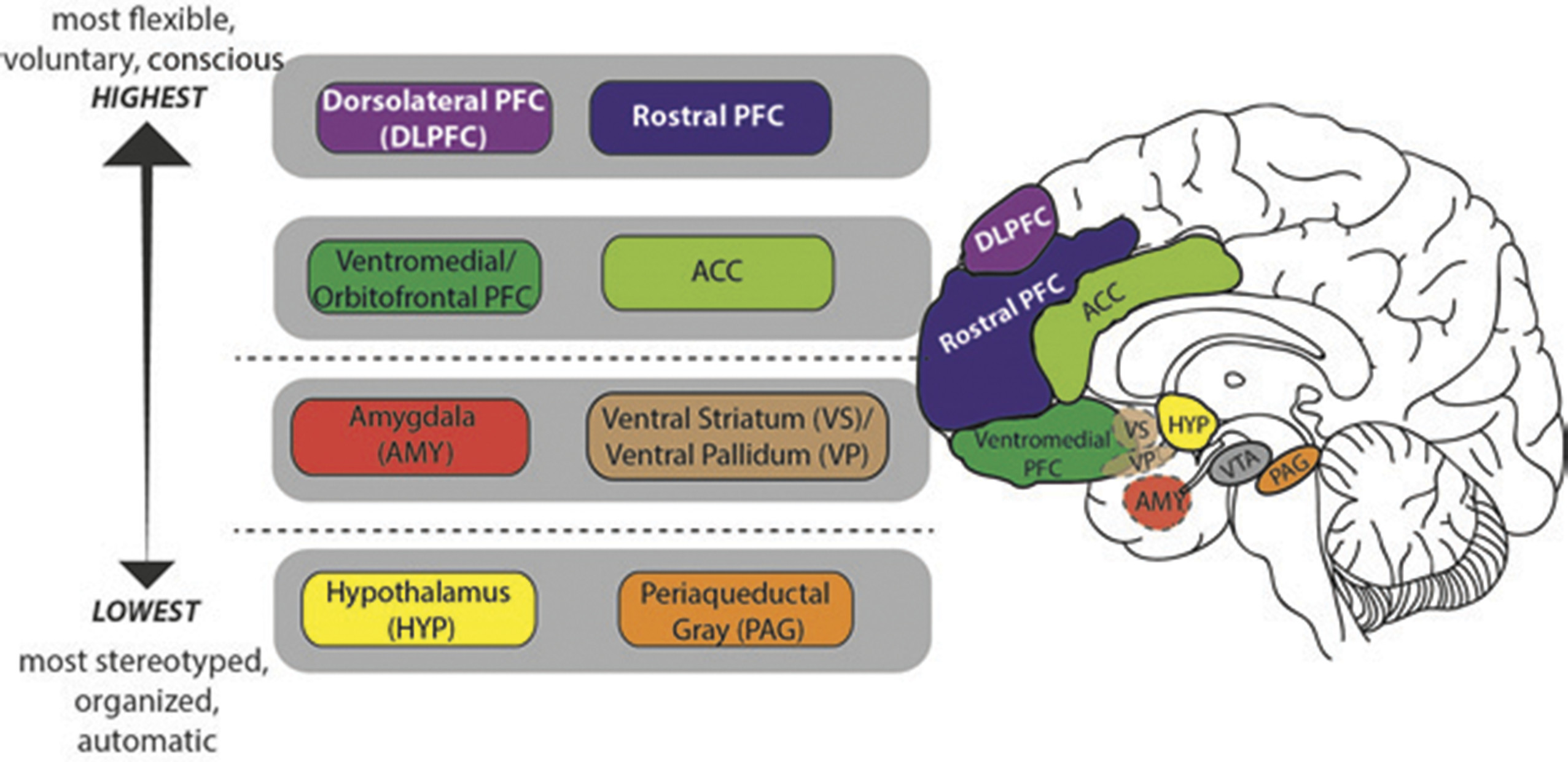
In humans, neural circuits underlying motivation/emotion form an integrated network that appears to function according to principles of hierarchic organization laid down by Hughlings Jackson in the late 19th century.
1 While certain details of his model have not withstood the test of time, this evolutionary scaffolding provides a helpful perspective from which to view the regions/functions involved in motivational processing and behavior. Jackson described the nervous system as an evolutionary accretion of functional levels ranging from the lowest, most organized, and most automatic to the highest, most flexible, and most voluntary. In keeping with Jackson’s model, motivational/emotional processing can be seen to originate at the level of the brainstem and incorporate layers of neural tissue of increasing differentiation and structural complexity during the course of phylogenetic and ontogenetic development.
2 As phylogenesis and ontogenesis unfold, the emotional system interacts increasingly with nonaffective cognitive processes, shaping the development of higher order adaptation to a wide range of stimuli and conditions (
Figure 2).
Key structures at the lower, diencephalic/mesenchephalic level include the hypothalamus and periaquaductal gray (PAG). The hypothalamus, which plays a major role in homeostatic regulation, coordinates somatic responses to changes in the internal milieu, or to stimuli initially processed at higher evaluative levels. It operates via a system of antagonistic excitatory and inhibitory nuclei that mediate production of preprogrammed adaptive behaviors, or fixed-action patterns. The stimulus-response associations mediated by the hypothalamus appear to be genetically determined, and modifiable only by descending inputs from higher levels of the network. The hypothalamus receives input from multiple sources including higher levels of the emotional/motivational network, visceral organs, circulating hormones, neurotransmitters, and osmotic components of the blood stream. Output targets the autonomic nervous system, pituitary gland/endocrine system, and subcortical motor centers that elicit stereotypic movements, and also affects higher level processing via noradrenergic, serotonergic, dopaminergic, and cholinergic projections.
The periaqueductal gray (PAG) is involved in defensive, reproductive and maternal behaviors, as well as arousal, analgesia, thermoregulation, vocalization, micturition, and regulation of REM sleep.
3 It receives input from higher levels of the emotional/motivational network including medial prefrontal cortex (PFC), anterior cingulate cortex (ACC), amygdala, and hypothalamus, as well as from nociceptive pathways. Output is to brainstem nuclei that coordinate specific patterns of cardiovascular, respiratory, motor and pain modulation, intralaminar and midline nuclei of the thalamus (relays to PFC), amygdala, hypothalamus, and substantia innominata.
Key structures at the intermediate, limbic level include the amygdala and ventral striatum/pallidum. While hypothalamic evaluative processes involve reference to genetically coded information, those at the level of the amygdala utilize information attained largely through experience, in the form of stimulus-reinforcement associations. Though most strongly associated with evaluation of threat, the amygdala responds to a broad array of significant and potentially significant (i.e., novel) stimuli.
4 It receives both interoceptive and exteroceptive input, the latter both directly from modality-specific thalamic relays, and following more complex cortical processing in primary sensory, unimodal and heteromodal association areas, and hippocampus. Output reaches higher and lower levels of the emotional/motivational network, the hippocampus and ventral striatum, sensory association cortex, and numerous effector regions.
The ventral striatum/pallidum, along with extended amygdala, can be viewed as linking motivation to action. While classically studied in the context of drug addiction or reward per se, their role extends to a broad range of positive stimuli/social rewards,
5 consistent with the conceptualization of happiness as an approach emotion.
6 The key structure is the nucleus accumbens (NAcc), which is comprised of a core and shell—the latter intimately related to the extended amygdala; the former to the dorsal striatum. More anterior regions of the nucleus accumbens show relative selectivity for rewards; more posterior regions, for losses.
7The ventral striatum also receives input from multiple levels of the emotional/motivational network, including hippocampus, basal amygdaloid complex, and limbic prefrontal cortex, and from midline and intralaminar thalamus, median raphe nuclei (serotonergic transmission), and the nucleus of the solitary tract (noradrenergic transmission). Outputs reach the ventral pallidum (to thalamus and frontal cortex), extended amygdale, lateral hypothalamus, basal forebrain cholinergic projection neurons, and dopaminergic neurons in the ventral tegmental area and substantia nigra.
Higher level processing involves paralimbic and prefrontal cortices. Like lower level components of the emotional/motivational network, with which they are interconnected, these regions receive exteroceptive and interoceptive input, and participate in the evaluation of significance and subsequent selection of action. Unlike lower level components, they are able to rapidly readjust behavioral responses to stimuli when their reinforcement value is changed, or when a more complex assessment of the current context suggests the need for modification.
8 These higher level regions, which receive top down cognitive and attentional modulation via input from dorsolateral and rostral PFC, are crucial for planning and sustaining goal-directed activity, anticipating consequences of behavior, and acting in accordance with socially determined norms.
While neurophysiologic studies have identified value-related signals in multiple brain regions, a large body of data indicates that orbitofrontal, ventromedial prefrontal, and anterior cingulate cortices play central roles in mediating motivationally guided stimulus evaluation and decision making.
9,10 Teasing out the role of specific frontal regions in the complex computations required to guide decision-making and goal-directed behavior is complicated by the fact that multiple processes, such as attention, arousal, motivation, and motor preparation correlate strongly with value, and the precise contributions of various regions is currently a matter of active investigation and hypothesis testing.
9–11 However, current evidence supports a number of conclusions, as reviewed by Kennerley and Walton.
10 Orbitofrontal cortex plays an important role in determining the current incentive value of a behavioral outcome, potentially influenced by current internal states, and in assigning the value of an outcome to the choice that produced that outcome. Ventromedial prefrontal cortex also plays a role in determining the current incentive value of a behavioral outcome, and in comparing alternative choices. Anterior cingulate cortex may integrate information about a decision’s expected value with information about an action’s value to determine the overall value of each choice alternative, and encodes reward prediction error signals. Lateral prefrontal regions also appear to track history of choices and outcomes, and to encode value information to support allocation of attentional resources or cognitive control toward behaviorally relevant information. In addition to their roles in motivated decision making, orbitofrontal and anterior cingulate cortices appear to mediate pleasure (“liking”).
9Dopaminergic neurons projecting from the ventral tegmental area to ventral striatal and prefrontal regions play a major role in reward processing, particularly the components of wanting and incentive learning or prediction. Rewarding stimuli initially stimulate phasic dopamine release in the nucleus accumbens, reinforcing appetitive behaviors. With repeated reinforcement, or habituation, rewarding objects lose the ability to stimulate dopamine release, while cues predictive of reward availability continue to do so, such that the signal is transferred to the cue. If reward is anticipated but not received, dopaminergic neurons decrease their firing rate. Thus, dopaminergic signals are involved in responding to novel or unanticipated rewards, anticipation or prediction of award, and reward-related learning, including monitoring of prediction errors, independent of reward modality.
12 The liking component of reward, in contrast, is most closely associated with opioid and endocannabinoid stimulation of hedonic “hot spots” found most notably in the shell of the nucleus accumbens and ventral pallidum.
13–15 There may also be a role for serotonin in modulating reward outcome value.
16Understanding the Motivational/Reward System: Foundations
Contributions to our understanding of the reward system come from a variety of fields. Major examples in the domain of animal studies include Heimer’s identification and mapping of the nucleus accumbens and ventral striatum,
17 McGinty’s investigations of molecular and neurochemical aspects of reward,
18 Haber’s elucidation of the structure and function of reward pathways,
19 Schultz’s work on dopaminergic mechanisms of reward prediction error and their relation to the computational-learning method of temporal difference learning,
12 and Gray’s Reinforcement Sensitivity Theory, which postulates a Behavioral Activation System that facilitates reactions to appetitive/rewarding stimuli and regulates approach behavior; a Fight-Flight-Freeze-System that mediates reactions to aversive/punishing stimuli and regulates avoidance behavior, and a Behavioral Inhibition System that mediates conflict within/between the Fight-Flight-Freeze and Behavioral Activation Systems.
20 Also crucial are Berridge and Robinson’s parsing of the circuitry and neurochemistry of wanting, liking and learning,
13 and development of the incentive sensitization theory of addiction,
21 and Rolls’ detailed neurophysiologic mapping of reward, especially in the orbitofrontal cortex,
9 and its relation to broader models of emotion, consciousness and computational neuroscience. More recently, a number of investigators have used optogenetic methods to expand our understanding of the distal circuit dynamics, extended inputs and outputs, and causal relationships underlying behavior in the mesolimbic dopamine system.
22 Within the human clinical/lesion literature, investigators such as Marin,
23 Robinson, Starkstein and Jorge,
24 Van Reekum,
25 Cummings,
26 Stuss,
25 and others have enhanced our understanding of apathy/amotivational syndromes, as discussed below.
The field of psychology has provided crucial research on traits related to reward. Major examples include Eysenck’s construct of extraversion
27; Watson, Clark, and Tellegen’s positive affectivity
28; and Depue and Collins’ agentic and affiliative modes of motivated behavior,
29 in which agentic behavior is driven by motivation/pleasure related to social dominance, leadership roles, assertiveness, and a subjective sense of potency in accomplishing goals, and affiliative behavior is driven by motivation/pleasure related to interpersonal warmth/affection (attachment). Behavioral economics/neuroeconomics is an interdisciplinary field that investigates human decision-making. Its origins lie in the application, by Kahneman and Tversky, of cognitive and social psychological approaches to economic decision-making,
30 giving rise to the field of behavioral economics; and the further application of cognitive neuroscience/neuroimaging approaches, referred to as neuroeconomics (e.g., Platt and Glimcher
31). Increasingly interdisciplinary, this field currently merges perspectives/approaches from cognitive and social psychology, experimental and behavioral economics, neuroscience, neuroimaging, theoretical biology, computer science, and mathematics (computational approaches). Important decision-related constructs from the field include utility, value, probabilistic and temporal uncertainty, risk, loss aversion, intertemporal choice (costs and benefits distributed over time), temporal discounting, and social decision making/game theory.
Translational/human studies, often involving concepts derived from the literatures described above, have also significantly enhanced our understanding of the motivational/reward system. The following examples provide a sense of their scope and intricacy. Using a monetary incentive delay task, Knutson et al.
32 demonstrated that anticipation of reward (versus nonreward) activated foci in the ventral striatum, whereas reward outcome was associated with activation in ventromedial prefrontal cortex. Peters and Buchel
33 observed correlations between the value of delayed rewards and activation in fronto-polar, lateral parietal, and posterior cingulate cortices; probabilistic rewards and activation in superior parietal and middle occipital regions; and domain-general coding of subjective value regardless of reward type in ventral striatum and orbitofrontal cortex. Similarly, Chib et al.
34 reported a correlation between activity in a specific region of ventromedial prefrontal cortex and subjects’ valuations of multiple categories of goods, suggesting an encoding of “common currency” that allows for shared valuation of different categories. Focusing on subjective pleasantness (“liking”) of food, Kringelbach et al.
35 found a correlation between decrease in subjective pleasantness and activation of orbitofrontal cortex when food is eaten to satiety, whereas Hare et al.
36 reported a correlation between activity in ventromedial prefrontal cortex and goal values in dieters engaged in real decisions about food consumption, regardless of amount of self-control. Investigating trait differences in tendencies to approach/avoidance, Pizzagalli et al.
6 showed an association between electroencephalographic activity in left dorsolateral and orbitolateral prefrontal cortex and increased bias to respond to reward-related cues, while Baik et al.
37 demonstrated a correlation between extraversion and dopaminergic receptor availability in the striatum using 18F-fallypride positron emission tomography.