Acute Effects and Insight into Reinforcing/Addictive Properties of Cannabis
All drugs of abuse increase DA release — a key neurobiological process that generates their reinforcing effects (
Koob and Volkow 2016). Here we evaluate the acute changes in DA circuitry associated with cannabis intake in preclinical and clinical studies that provide basis for the reinforcing effects of cannabis. While the two main constituents of cannabis are delta9-tetrahydracannabinol (THC) and cannabidiol (CBD), THC seems to be responsible for cannabis’ addictive potential due to its psychoactive properties and associated effects on brain dopaminergic function. Acute THC administration elicits striatal DA release in animals (
Ng Cheong Ton et al. 1988) and humans (
Stokes et al. 2010;
Bossong et al. 2015;
Bloomfield et al. 2016). However, another study found no evidence for THC-induced DA release (
Barkus et al. 2011); this may be because THC induces quantitatively less DA release than psychostimulants such as methylphenidate or amphetamine (
Volkow et al. 1999a). Nonetheless, these findings suggest that THC increases DA release similar to other drugs of abuse.
Several animal models of cannabis exposure have been established in rodents and non-human primates (
Panlilio et al. 2015). In studies with rodents, neurophysiological methods such as intracranial microinjection, microdialysis, and single-unit electrophysiological recording techniques are used to study the acute effects of THC and other cannabinoids in the brain directly (
Oleson and Cheer 2012;
Panlilio et al. 2015). Behavioral methods include the use of place conditioning, drug discrimination, intracranial self-stimulation, or intravenous self-administration to study the reinforcing effects of cannabinoids in vivo (for further details see:
Maldonado and Rodriguez de Fonseca 2002;
Tanda and Goldberg 2003;
Maldonado et al. 2011;
Panlilio et al. 2015;
Zanda and Fattore 2018). Robust intravenous self-administration paradigms in animals have been difficult to establish. That is, in rodents THC is unable to sustain intravenous selfadministration (
Lefever et al. 2014), whereas squirrel monkeys have found to self-administer THC; suggesting differences in species. However, other behavioral methodologies, such as drug discrimination and conditioned place preference paradigms, reveal the rewarding effect of THC and other cannabinoids (
Maldonado and Rodriguez de Fonseca 2002;
Tanda and Goldberg 2003;
Maldonado et al. 2011;
Oleson and Cheer 2012;
Panlilio et al. 2015).
In rodents, THC-induced DA release is associated with increased intracranial self-stimulation in key reward pathways of the brain (
Katsidoni et al. 2013). Likewise, low doses of a cannabinoid-1 receptor (CB1R) agonist in the PFC increased spontaneous firing and bursting rates of ventral tegmental area (VTA) DA neurons, which was associated with potentiated salience of fear memories in rats (
Draycott et al. 2014). THC elicits striatal DA release by activating CB1R, which are colocalized with DA receptors in the striatum and substantia nigra, regions implicated in salience processing (
Wijayendran et al. 2016). This suggests that the endocannabinoid system (eCS) is involved in regulating DA release during salience attribution (
Bloomfield et al. 2016), and that acute THC dysregulates the dopaminergic and endocannabinoid systems which then leads to impairments in salience processing (
Wijayendran et al. 2016). These preclinical findings may provide a biological basis for human studies which show impaired salience processing after THC administration. In one study, THC-potent cannabis was found to increase attentional bias towards cannabis-related stimuli in cannabis users during a computer-based dot-probe behavioral task (
Morgan et al. 2010). In a separate fMRI task, healthy participants performed a visual oddball paradigm; THC administration resulted in making non-salient stimuli appear more salient (
Bhattacharyya et al. 2012). Together, these pre-clinical and clinical findings reveal that THC administration has reinforcing properties that alter salience processing via increased dopaminergic signaling like other drugs of abuse (
Morgan et al. 2010;
Bhattacharyya et al. 2012;
Draycott et al. 2014;
Wijayendran et al. 2016;
Bloomfield et al. 2016).
Long-Term Effects of Cannabis: Behavior and Cognition
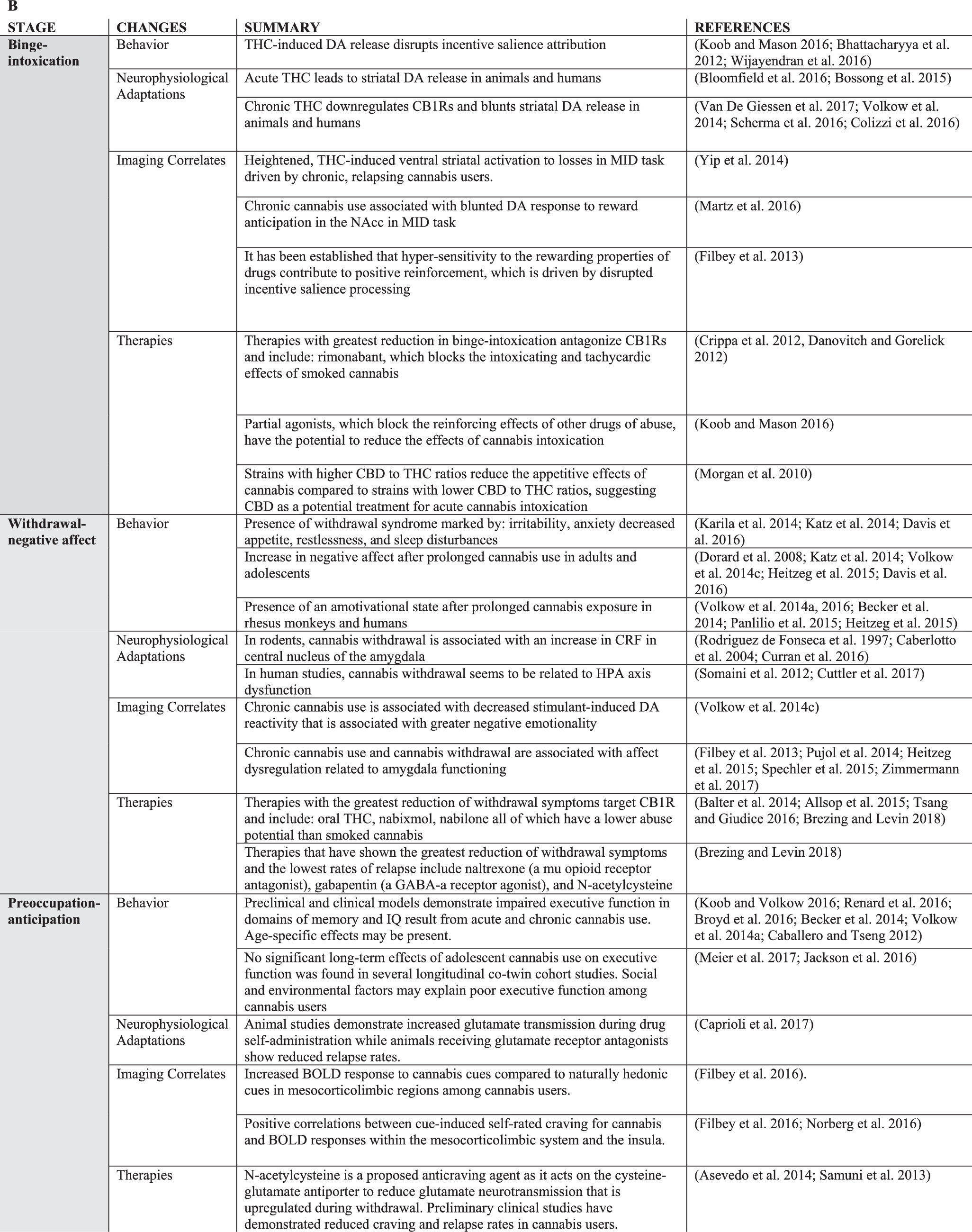
Chronic cannabis use is associated with an increased risk of developing substance use disorders (SUD); about 9% of those who use cannabis present with characteristic symptoms of dependence according to DSM-IV criteria (
Volkow et al. 2014a). Diagnoses of cannabis abuse and dependence in the DSM-IV did not include withdrawal due to uncertainty of its diagnostic features (
Katz et al. 2014) In the DSM-5, however, cannabis abuse and dependence fall under a diagnosis of CUD which now includes withdrawal from cannabis. Withdrawal was added as a diagnostic criteria for CUD as it is often accompanied by increased functional impairment of normal daily activities similar to those seen in other SUD (
Karila et al. 2014;
Katz et al. 2014;
Davis et al. 2016). Symptoms of cannabis withdrawal also seem to appear in a similar time course and manner as withdrawal from other substances (
Karila et al. 2014).
A clinical diagnosis of cannabis withdrawal includes irritability, anger or aggression, nervousness or anxiety, sleep difficulty, decreased appetite or weight loss, restlessness, depressed mood, and physical symptoms causing significant discomfort such as shakiness or tremors, sweating, fever, chills, and headaches (
Karila et al. 2014;
Katz et al. 2014). Typically, symptoms of cannabis withdrawal occur 1 to 2 days after cessation of heavy use and can last between 7 and 14 days (
Davis et al. 2016). The most common symptoms observed during cannabis withdrawal include irritability, anxiety, decreased appetite, restlessness, and sleep disturbances (
Oleson and Cheer 2012;
Panlilio et al. 2015;
Curran et al. 2016;
Gates et al. 2016). Sleep disturbances seem to be characterized by trouble falling asleep, decrease in total sleep time, and the presence of nightmares and strange dreams (
Gates et al. 2016). The severity of withdrawal symptoms was associated with greater negative impact on normal, daily activities (
Davis et al. 2016) suggesting that the effects of cannabis withdrawal seem to parallel withdrawal in other drugs of abuse.
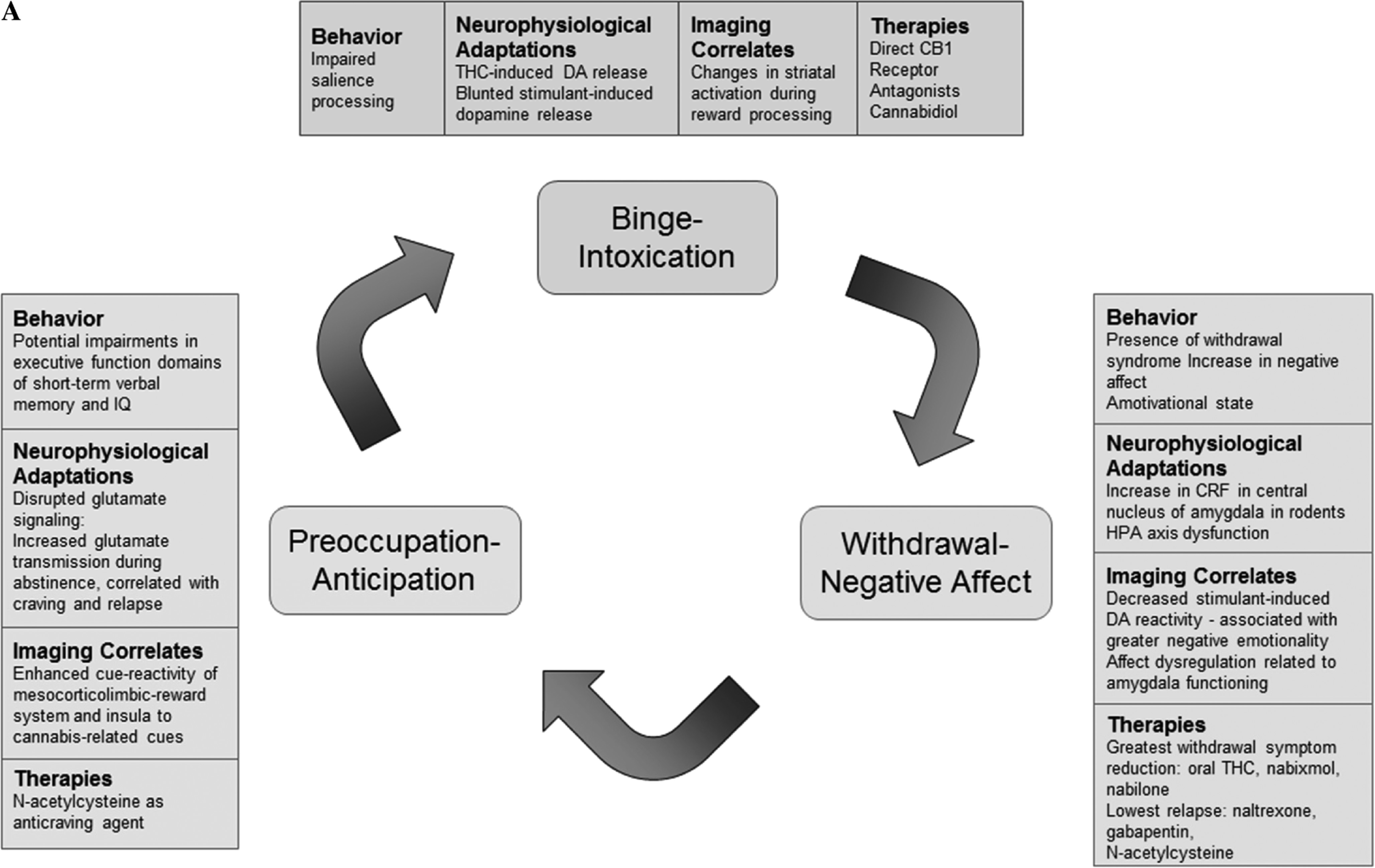
Koob and Volkow (2016) posit that the withdrawal stage of addiction is marked by an increase in negative affect which also seems to be the case for cannabis addiction (
Volkow et al. 2014c). In addition to acute withdrawal-related emotional disturbances such as irritability and anxiety (
Katz et al. 2014;
Davis et al. 2016), prolonged cannabis use is associated with long-term affect dysregulation. In a longitudinal study of adolescents, cannabis users consistently reported greater negative emotionality than healthy controls between the ages of 13 and 23; moreover, as healthy controls showed a decrease in negative emotionality with age, negative emotionality remained elevated for cannabis users during over the same time (
Heitzeg et al. 2015). Another study of adolescents found that half of a group of adolescents undergoing treatment for cannabis withdrawal had at least one comorbid diagnosis of anxiety or depression; additionally, for these adolescents greater cannabis use was associated with increased depressive and anxiety-like symptoms (
Dorard et al. 2008).
These changes in the affective state after prolonged cannabis use may also influence motivation. In both rhesus monkeys and humans, withdrawal from cannabis seems to involve the presence of an amotivational state (
Karila et al. 2014;
Panlilio et al. 2015;
Volkow et al. 2014a,
b,
c,
2016). The amotivational state has been previously described as a "reduced motivation and capacity for usual activities required for everyday life, a loss of energy and drive to work and personality deterioration" (
Karila et al. 2014). The origin of this amotivational state is still unknown and may be related to changes in executive function (
Karila et al. 2014) and to reduced dopamine signaling after chronic cannabis use (
Bloomfield et al. 2014;
Volkow et al. 2014c). In rhesus monkeys, chronic cannabis smoke exposure was associated with lower motivation scores in a place conditioning paradigm, although these effects disappeared two to three months after cessation of the cannabis treatment (
Paule et al. 1992). In one study of neurocognition, chronic cannabis users demonstrated impairments in verbal memory, spatial working memory, spatial planning, and motivated decision-making compared to healthy controls (
Becker et al. 2014). These findings suggest that the amotivational state during withdrawal may be related to cognitive dysfunction and to reduced dopamine signaling after chronic cannabis use.
Cognitive dysfunction, specifically impairments in executive domains, after chronic cannabis use is a key feature of the neurobiological model of addiction (
Koob and Volkow 2016). Deficits in executive function after chronic cannabis use have been shown in both preclinical and clinical studies. In one preclinical study, chronically administering a synthetic cannabinoid agonist to adolescent rats impaired short-term working memory in adulthood (
Renard et al. 2016). Specifically, this chronic cannabinoid exposure altered PFC structure and impaired cortical synaptic plasticity from reduced long-term potentiation (LTP) in the hippocampus-PFC circuit. These findings support the theory that adolescent cannabis use causes lasting deficits in memory. However, they are likely agespecific effects as preclinical and clinical studies have demonstrated a lack of long-lasting cognitive impairments from adult chronic cannabis use (
Renard et al. 2016).
Many clinical studies have investigated the long-term effects of chronic cannabis use on markers of executive function such as IQ, verbal learning, and memory. The results are varied and equivocal, as longitudinal studies with controlled confounds are difficult to establish.
Volkow et al. (2014a,
b,
c) report that cannabis use during adolescence and young adulthood is associated with impaired functional connectivity in the brain and corresponding declines in IQ. A 2016 systematic review of 105 papers assessing the acute and chronic effects of cannabis on human cognition found that memory has been the most consistently impaired cognitive measure (both after acute and chronic cannabis use), with the strongest effects in the verbal domain (
Broyd et al. 2016). The evidence for impairments in other domains of executive function such as reasoning, problem solving, and planning was less conclusive, as numerous studies found no significant differences in casecontrol comparisons. However, studies examining heavy users as well as early-onset users reported impaired executive function, especially when the sample was predominantly older participants (
Becker et al. 2014;
Broyd et al. 2016). This may suggest a conditional effect, unique to adolescent and heavy cannabis users while moderate and adult users are less vulnerable to the harmful effects of cannabis on cognition.
Despite earlier findings of impaired executive functioning in adolescent- and early- onset users, it is important to note that several recent studies found no significant long-term effects of adolescent cannabis use on executive function.
Meier et al. (2018) report a longitudinal co-twin control study that showed no significant association between adolescent cannabis use and neuropsychological decline, and instead suggest social and environmental factors as explanations for poor executive function among cannabis users. This study was particularly insightful because of a large sample size (
n = 1989) and IQ assessments prior to the onset of cannabis use (IQ obtained at age 5, 12, and 18). It demonstrated that adolescents who used cannabis had a lower childhood IQ and a lower IQ at 18 than non-users, but that there was no decline in IQ from pre- to post-cannabis use (
Meier et al. 2018). These results are in line with another co-twin longitudinal study that investigated two large cohorts of twins and found no significant difference in IQ change over time between twins discordant for cannabis use (
Jackson et al. 2016). However, lower baseline IQ was associated with adolescent cannabis use suggesting that social and environmental factors influence an adolescent’s subsequent cannabis use (
Jackson et al. 2016). Together, these studies suggest that lower IQ may be a risk factor for cannabis abuse rather than the use of cannabis resulting in neuropsychological decline. However findings on the effects of cannabis exposure during adolescents are controversial and require investigation with prospective designs that take advantage of brain imaging technologies. The ABCD study, a prospective study that aims to follow 10,000 children as they transition into adulthood with a detailed phenotypic characterization including periodic brain imaging, would help clarify what effects cannabis consumption might have on brain development, neurocognitive function and mental illness (
Volkow et al. 2017b).
Long-Term Effects of Cannabis: Neurophysiological Changes
The chronic relapsing nature of addiction seems to involve underlying neurophysiological changes in reward, stress, and executive function circuits (
Koob and Volkow 2016). Here we summarize findings about the effects of chronic cannabis use on these circuits.
In rats, early-life exposure to THC blunts dopaminergic response to naturally rewarding stimuli that elicit DA release later in life (
Bloomfield et al. 2016). Likewise in rats, adolescent exposure to THC resulted in increased self-administration of and blunted striatal DA response to CB1R agonists in adulthood (
Scherma et al. 2016). Changes in reward-related circuitry after chronic cannabis use may be related to changes in the eCS after prolonged cannabis use. The eCS has been implicated in reward-processing and reward-seeking behavior given that CB1 receptors are densely expressed in areas associated with reward processing and conditioning including the amygdala, cingulate cortex, PFC, ventral pallidum, caudate putamen, NAcc, VTA, and lateral hypothalamus (
Parsons and Hurd 2015;
Volkow et al. 2017a). In animals, activation of CB1 receptors seems to influence the hedonic effects of natural rewards after THC administration, suggesting that cannabis can affect reward sensitivity via activation of CB1 receptors (
Parsons and Hurd 2015).
Chronic THC exposure has further been shown to down-regulate CB1Rs, providing a neurobiological basis for the development of tolerance and desensitization to the rewarding effects of THC (
Colizzi et al. 2016). In rodents, chronic administration of THC or CB1R agonists leads to tolerance in most responses as well as a decrease in CB1R availability in many brain areas (
Maldonado and Rodriguez de Fonseca 2002;
Tanda and Goldberg 2003;
Maldonado et al. 2011). In cannabis users, withdrawal symptoms have also been associated with reductions in CB1R availability as assessed by [
11C]OMAR PET imaging (
Curran et al. 2016;
D’Souza et al. 2016).
Hirvonen et al. (2012) found that cannabis use downregulates CB1R in cortical regions, potentially altering the brain’s reward system. However, they also found that after 4 weeks of abstinence, CB1R density returned to normal in cannabis users in all regions except the hippocampus. This suggests that some neurobiological changes of chronic cannabis use are reversible (
Hirvonen et al. 2012).
Chronic cannabis use and administration is also associated with neurophysiological changes in stress responsivity. In rodents, the neurophysiological changes associated with cannabis withdrawal are modeled through precipitated withdrawal through the use of rimonabant (a selective CB1R blocker) after repeated cannabinoid treatment (
Maldonado et al. 2011;
Oleson and Cheer 2012;
Panlilio et al. 2015). Cannabinoid withdrawal in rodents is associated with an increase in the stress peptide CRF in the central nucleus of the amygdala (
Rodriguez de Fonseca et al. 1997;
Maldonado et al. 2011;
Panlilio et al. 2015;
Curran et al. 2016), which suggests the presence of between-systems changes in brain stress systems, as described by the Koob and Volkow model (2016). In addition, the eCS seems to be involved in regulating the stress response through its action on the amygdala and HPA axis (
Dow-Edwards and Silva 2017;
Volkow et al. 2017a). The eCS modulates interactions between the PFC, amygdala, and hippocampus which are all involved in emotional memory, anxiety-related behaviors, and drug cue-induced craving in SUD (
Jasinska et al. 2014). Additionally, endocannabinoids seem to be required for feedback to normal stress responses: glucocorticoids increase the endogenous cannabinoids anandamide (AEA) and 2-acylglycerol (2-AG) in the paraventricular nucleus while CB1R antagonists increase HPA axis output. In rodents, exogenous cannabinoids seem to create a dysregulation of stress responsivity and anxiety-related behaviors (
Dow-Edwards and Silva 2017).
Moreover, chronic cannabis abuse is associated with the dysregulation of stress responsivity in humans (
Curran et al. 2016). Studies in cannabis users show that chronic cannabis use is related to both blunted and hyperactive stress responses (
Somaini et al. 2012;
Cuttler et al. 2017).
Cuttler et al. (2017) found that healthy controls had an increase in cortisol levels under a stress-provoking condition compared to baseline but did not find the same increase in active cannabis users. In another study, both active and abstinent cannabis users had persistent hyperactivity of the HPA axis (measured by blood cortisol and ACTH levels) compared to healthy controls (
Somaini et al. 2012). This pattern of HPA axis dysregulation is also seen in alcohol users: chronic alcohol use seems to attenuate the cortisol response to acute psychological stimulation of the HPA axis, but is related to elevated cortisol levels during alcohol intoxication and abstinence in dependent users (
Stephens and Wand 2012).
In addition to its role in HPA axis dysfunction and reward processing, the hyperactivation of the eCS may also play a role in the executive dysfunction sometimes observed in cannabis use. The eCS is highly active in adolescent brain development, particularly in the PFC, a region that exercises executive function (
Dow-Edwards and Silva 2017). Exogenous cannabinoids hyperactivate CB1 receptors which are expressed in pyramidal neurons and GABAergic interneurons, indicative of the regulatory role of the eCS in GABA and glutamate neurotransmission (
Caballero and Tseng 2012;
Volkow et al. 2017a). Activation of presynaptic CB1 receptors inhibits glutamate transmission onto GABAergic cells in the PFC, reducing the function of inhibitory prefrontal circuits. Therefore, hyperactivation by exogenous cannabinoids during development could disrupt the maturation of GABAergic interneurons in the PFC and desynchronize PFC circuits (
Caballero and Tseng 2012). Thus, adolescent cannabis use may affect brain development and result in enduring alterations in the GABA/glutamate balance in the PFC (
Renard et al. 2016).
Neuroadaptations in glutamatergic signaling resulting from repeated cannabis use are likely also implicated in periods of cannabis abstinence and craving (
Samuni et al. 2013). This theory is supported by a review of animal studies that demonstrated increased glutamate signaling during drug self-administration and relapse, offering a potential neurochemical target for treatment in preventing craving and subsequent relapse. For example, rodent and nonhuman primate models receiving periodic injections of glutamate receptor antagonists have shown a reduction in relapse rates (
Caprioli et al. 2017). Nonetheless, these findings need to be corroborated in rodents since there is conflicting evidence for whether self-administration in rodent models provides robust evidence of THC as a behavioral reinforcer (
Tanda and Goldberg 2003;
Maldonado et al. 2011;
Panlilio et al. 2015;
Melis et al. 2017).
Long-Term Effects of Cannabis on the Brain: Neuroimaging Studies
Addiction is a recurring cycle that worsens over time and involves neuroplastic changes in the brain reward, stress, and executive function systems (
Koob and Volkow 2016). Previous neuroimaging studies reveal the long-term effects of chronic cannabis use on several different brain systems including the reward, endocannabinoid, and stress systems as well as brain areas involved in emotion processing and decision making.
Similar to animal models of chronic THC exposure, chronic cannabis use has been shown to blunt DA response to DA releasing stimulant drugs in the striatum with both [
11C]-(+)-PHNO and [
11C]raclopride PET imaging (
Volkow et al. 2014c;
Bloomfield et al. 2016;
van de Giessen et al. 2017) and to decrease DA synthesis as assess with PET imaging with [
18F]DOPA (
Bloomfield et al. 2014) (
Fig. 2). This pattern of decreased stimulant-induced DA release is also seen with chronic use of other drugs of abuse such as alcohol, cocaine, and nicotine (
Koob and Volkow 2016). However, cannabis users do not show lower baseline D2/D3 receptor availability in the striatum compared to healthy controls – a pattern seen in chronic alcohol, nicotine, cocaine, opiate and methamphetamine users (
Volkow et al. 1996b,
2001,
2002,
2014b,
2017c;
Wang et al. 1997;
Martinez et al. 2012;
Tomasi et al. 2015b;
Wiers et al. 2016a,
2017;
Ashok et al. 2017). Moreover, the stimulant challenge led to significantly lower self-reported ratings offeeling high (
Volkow et al. 2014c), and decreased brain glucose metabolism in the striatum, thalamus, and midbrain (
Wiers et al. 2016b) in cannabis users versus controls. Cannabis users had higher negative emotionality and lower positive emotionality personality scores than controls, and negative emotionality scores were inversely correlated with methylphenidate-induced dopamine increases in the ventral striatum (
Volkow et al. 2014c;
Wiers et al. 2016b). These findings offer an explanation for decreased dopamine reactivity in the striatum during abstinence that may contribute to negative emotionality, which is consistent with lower reward sensitivity in cannabis users during the withdrawal phase of addiction (
Volkow et al. 2014c). In another study, a stimulant challenge also led to blunted brain glucose metabolism in striatal regions, which was associated with craving (
Wiers et al. 2016b). Together these findings from stimulant challenges indicate functional changes in the dopaminergic reward system in chronic cannabis users.
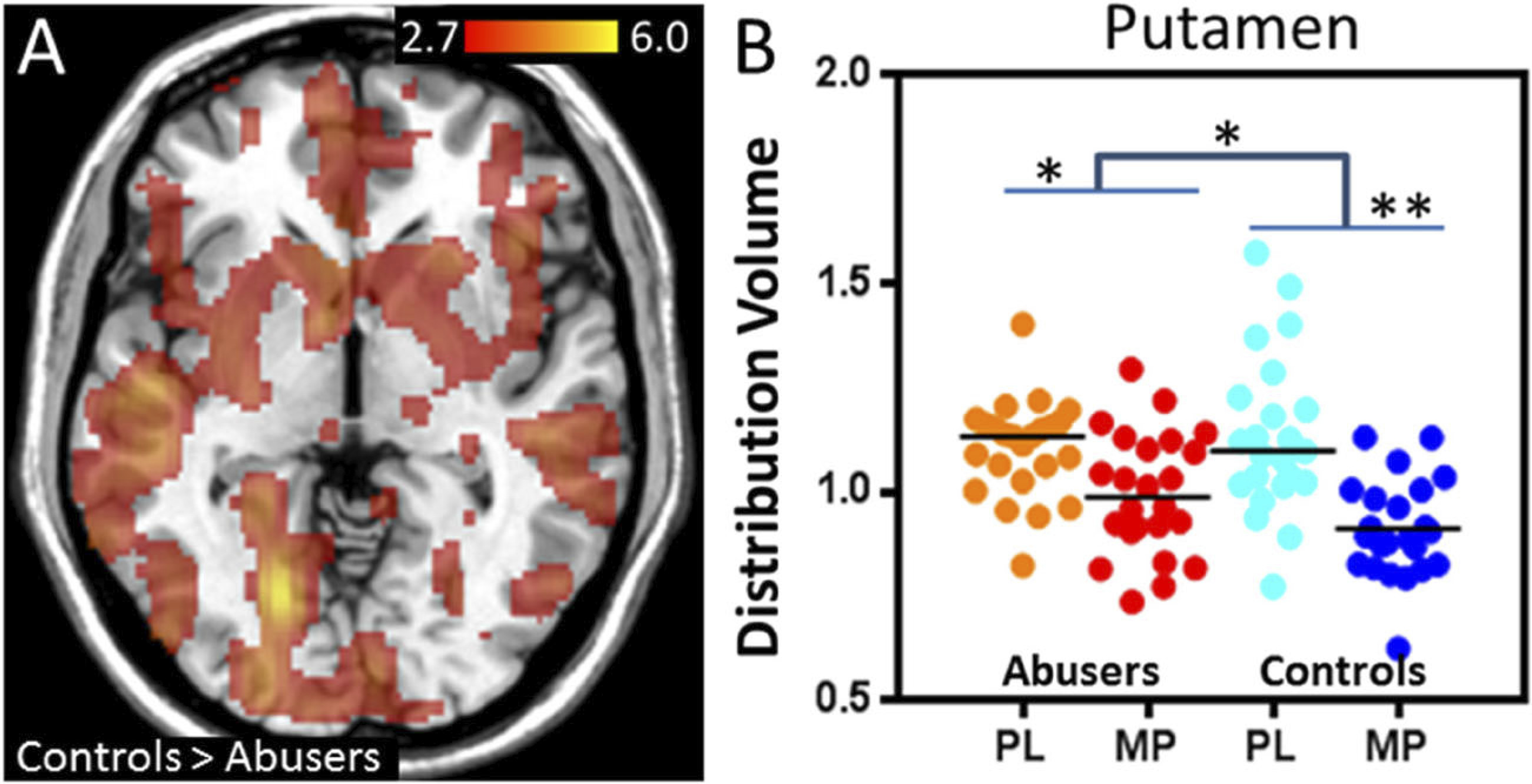
Furthermore, fMRI studies have also revealed functional and structural changes in brain areas involved in reward processing after chronic cannabis use. In one study, participants in a cannabis-dependent group had greater activation in the ventral striatum in response to losses during a monetary incentive delay (MID) task compared to healthy controls (
Yip et al. 2014). Compared to controls, the cannabis-dependent participants also had smaller putamen volumes, a brain region involved in habit formation. These differences seemed to be driven by participants who were unable to stay abstinent from cannabis and were comparable to findings in tobacco smokers suggesting similar changes in reward functioning in both tobacco and alcohol addiction (
Yip et al. 2014). In another fMRI study with the MID task, cannabis users in withdrawal had greater activation in the ventral striatum in response to positive incentives compared to healthy controls during the MID task, similar to findings in alcohol users (
Filbey et al. 2013). Persistent cannabis use also seems to be related to a blunted response to reward anticipation in the NAcc during the MID task: in this study, even after controlling for prior and current use of other drugs, greater cannabis use was related to decreased activation in the NAcc during reward anticipation at baseline, 2 year, and 4 year follow ups (
Martz et al. 2016). Together, these findings suggest that chronic cannabis use produces functional alterations in areas involved in reward processing.
A recent fMRI study investigated whether cannabis use sensitizes and disrupts the mesocorticolimbic reward processes during a hedonic cue-reactivity task. A cohort of chronic cannabis users (requiring 72 h of abstinence) showed greater BOLD response for cannabis cues compared to natural reward cues (fruit) in the orbitofrontal cortex (OFC), striatum, anterior cingulate gyrus, and VTA, regions along the mesocorticolimbic-reward pathway (
Filbey et al. 2016). In cannabis users, there were also significant positive correlations between cue-induced self-rated craving for cannabis and BOLD responses within the mesocorticolimbic system and in the insula. The latter data supports the addictive model of cannabis as insula activation may serve as a biomarker to help predict relapse (
Filbey et al. 2016). This brain region contributes to interoceptive awareness of negative emotional states and is differentially activated during craving (
Koob and Volkow 2016). This is also consistent with prior findings that the dopaminergic reward system is reactivated during acute craving episodes (
Volkow et al. 1999b,
2005;
Koob and Volkow 2016). Moreover, in cannabis abusers, but not in controls, acute THC intoxication elicited activation of brain reward regions as assessed by increases in brain glucose metabolism in striatum and orbitofrontal cortex (
Volkow et al. 1996a). Overall, these studies demonstrates that chronic cannabis use sensitizes the mesocorticolimbic-reward system to cannabis cues and to THC (
Volkow et al. 1996a;
Filbey et al. 2016). These findings suggest that chronic cannabis use affects key brain circuits involved in the reward system similar to other drugs of abuse.
In addition to changes in reward processing, chronic cannabis use also seems to affect emotion processing. Several MRI studies reveal functional and structural differences in the amygdala – a key brain structure in processing emotions – after chronic cannabis use. Compared to healthy controls, adolescents who used cannabis had lower activation in the amygdala in an emotional arousal word task during fMRI (
Heitzeg et al. 2015). However, in another fMRI study, adolescent cannabis users showed greater amygdala activation to angry faces compared to controls (
Spechler et al. 2015). Another study of facial emotion recognition found that during abstinence, cannabis-dependent patients performed significantly worse than controls in the identification of negative emotions suggesting a lasting impact on emotion recognition after chronic cannabis use (
Bayrakçi et al. 2015). Together, these fMRI findings indicate that chronic cannabis use alters amygdala function.
The association between amygdala structure and cannabis use is relatively unclear. Some studies have found morphological and volumetric differences in the amygdala between healthy controls and cannabis users in both adolescent and adult cohorts (
Gilman et al. 2014;
Lorenzetti et al. 2015). On the other hand, other studies that controlled for alcohol and tobacco use found no differences in amygdala volume or shape between cannabis users and healthy controls (
Weiland et al. 2015;
Manza et al. 2018). A longitudinal study with cannabis users and healthy controls found no volumetric differences in gray matter at baseline or a three-year follow up (
Koenders et al. 2016). Despite these inconclusive structural MRI findings, there is evidence that chronic cannabis use may contribute to emotional dysregulation through functional changes in the amygdala (
Heitzeg et al. 2015;
Spechler et al. 2015).
Further evidence of emotion dysregulation after chronic cannabis use is seen in fMRI functional connectivity studies with cannabis users (
Pujol et al. 2014;
Zimmermann et al. 2018). In one study, cannabis users showed increased resting-state functional connectivity between posterior cingulate cortex (PCC) and other regions of the default mode network (including angular gyri, medial and lateral PFC, ACC and temporal cortex), and an anticorrelation between PCC activation and insula activation. These connectivity patterns were associated with a reduction in anxiety scores suggesting an alteration of affect state that is related to changes in brain function during cannabis addiction. As the insula is involved in integrating interoceptive information for emotion, these findings suggest that cannabis may enhance visceral sensations via insula activation to modify affect state (
Pujol et al. 2014). Additionally, these resting-state functional connectivity patterns lasted one month after cessation of cannabis use suggesting long-lasting changes in brain function after chronic cannabis use (although functional connectivity patterns in other networks normalized with abstinence, see
Pujol et al. 2014). In another fMRI study, cannabis-dependent subjects completed task and resting state fMRI 28 days after abstinence (
Zimmermann et al. 2018). During the task, in which participants were passively exposed to pictures of negative and neutral valence, negative emotional stimuli elicited larger increases in medial orbitofrontal cortex (mOFC) activity in cannabis-dependent users than in healthy controls; researchers also found greater functional connectivity between the mOFC and dorsal striatal region as well as the mOFC and amygdala compared to healthy controls during the task. Given that the mOFC is a region implicated in emotional regulation, these connectivity findings suggest the existence of persistent emotional processing alterations in cannabis-dependent users even after cessation of cannabis use (
Zimmermann et al. 2018).
In addition to contributing to emotion dysregulation, cessation of chronic cannabis use is associated with the development of craving (
Davis et al. 2016). Cue-reactivity is a neurobiological metric to evaluate cue-induced craving, a strong predictor of relapse for substance use (
Budney et al. 2008;
Wilson and Sayette 2015). A 2016 meta-analysis of cue-reactivity in regular cannabis users reported moderate to extreme cue-reactivity despite self-reports of low craving (
Norberg et al. 2016). These results may indicate that cannabis users underestimate their own excessive salience, suggesting that self-reports may not accurately reflect cannabis craving intensity. Thus, excessive salience attribution to cannabis-related cues appears to be a hallmark of cannabis addiction. These studies further demonstrate the importance of collecting objective measures of craving when studying the effects of chronic cannabis use.
Finally, one of the most consistent neuroimaging findings in addiction is that of dysregulation of frontal cortical regions involved with executive function including the dorsolateral prefrontal cortex, the ACC and the inferior frontal cortex. Imaging studies investigating brain glucose metabolism, which serves as a marker of brain function, reported decreased frontal metabolism in cannabis abusers when compared with controls (
Sevy et al. 2008;
Wiers et al. 2016b) and in polysubstance users who consumed cannabis (
Moreno-Lopez et al. 2012).