It is well established that epilepsy is associated with an increased risk of various psychopathologies, including depression, bipolar disorder, and psychotic disorders.
1,2 Psychogenic non-epileptic seizures (NES) also frequently coexist with psychiatric illnesses, such as anxiety, depression, posttraumatic stress disorder (PTSD), suicidal ideation/attempts, or other psychological disorders.
3 Also, individuals with temporal lobe epilepsy (TLE) and NES are at increased risk for personality disorders.
One study, using a structured psychiatric interview, found that NES patients were more likely to exhibit borderline personality traits, whereas patients with epilepsy were more likely to have avoidant personality traits.
4 Other studies have shown obsessive traits and “overly-controlled personality” in patients with NES,
5,6 factors that may influence the underlying psychopathology of NES. There is an extensive historical literature on personality characteristics in TLE (i.e., interictal personality disorder), although the issue is controversial.
7,8 The concept of a distinct interictal behavioral syndrome in TLE was initially described by Gastaut
9 and named by Geschwind and Waxman.
10 Characteristic traits included changes in sexual behavior and aggression, increased philosophic and religious concerns, viscosity, and compulsive writing (often referred to as the “Gastaut-Geschwind” syndrome).
In response to lack of evidence for this syndrome with traditional measures of personality and psychopathology (e.g., MMPI, Rorschach), Bear and Fedio developed a scale (Bear-Fedio Inventory [BFI]) measuring 18 proposed TLE behavioral traits and administered the scale to individuals with TLE, healthy control subjects, and individuals with neuromuscular disorders.
11 They found that TLE patients endorsed more traits than both healthy-controls and the medical-contrasting group. The traits that were most discriminating included deepened emotions, circumstantiality, altered religious and sexual concerns, and hypergraphia. After Bear and Fedio's publication, a number of studies investigated the BFI instrument and the hypothesis that these personality and behavioral traits were specific to TLE. By and large, studies support the notion that individuals with TLE exhibit increased behavioral traits on the BFI as compared with healthy-controls and other medical groups. There is also recent work showing that individuals with TLE and bilateral hippocampal atrophy endorse more behavioral traits than those with epilepsy and no-atrophy.
12 Similarly, Trimble and Freeman found that individuals with epilepsy and increased religiosity endorsed hypergraphia, greater emotionality, and increased philosophical ideas than individuals with TLE with no religiosity.
13 There remains significant controversy about whether the syndrome is distinctive to TLE, given mixed findings when comparing individuals with TLE and patients with psychiatric illness or individuals with generalized epilepsy. Several authors suggest that the BFI measures general psychopathology, rather than a specific TLE syndrome.
14,15 Shetty and Trimble carefully reviewed findings from past studies and concluded that most studies support the original Bear and Fedio results that the BFI can differentiate between TLE and other healthy, neurologic, or psychiatric groups. They argued that evidence supports a distinct TLE behavioral syndrome.
16 They also suggest that the most consistent traits seem to match up with the original Geschwind syndrome (i.e., religiosity, hypergraphia, hypermoralism), suggesting that further refinement of the scale might be useful. Although only a minority of patients develop these syndromes, at least a subset of individuals with TLE exhibit the characteristic interictal personality traits. Whether these traits are truly specific to TLE remains an open question. The current study examines differences between patients with TLE and NES on the BFI. To-date, no studies have been published describing the BFI in individuals with NES. Understanding interictal personality traits in NES may help differentiate patients with TLE and NES in clinical evaluation. It is also of theoretical interest because both patient groups experience seizure behavior, but only the TLE group has pathologic electrical activity in limbic structures. Given previous BFI study results, we hypothesized that patients with TLE would endorse more symptoms on the BFI than those with NES.
METHOD
Subjects and Procedure
Participants: included 41 video EEG-confirmed patients with TLE and 37 video-confirmed patients with NES, who were seen as part of their clinical treatment in a tertiary-care academic hospital. Individuals with TLE came from consecutive referrals to a neuropsychology outpatient practice or were identified during consecutive inpatient admissions for EEG monitoring. NES patients were consecutive clinical referrals to one of the authors of the study (WCL) or were identified during consecutive inpatient admissions for EEG monitoring. The diagnosis of NES was defined as stereotypic, motor manifestations (including the initiation or cessation of motor activity/staring), with or without change in level of consciousness. On video EEG monitoring, there was no recognizable build-up of rhythmic epileptiform (ictal) activity immediately before, during, or after the event. On the basis of EEG findings, 13 TLE patients had primarily right-sided abnormalities, 21 had left-sided findings, and 7 had bilateral abnormalities. Exclusion criteria included full-scale IQ <75, history of whole-brain irradiation, or history of other active neurological disorder.
Measures
All participants completed the Bear-Fedio Inventory (BFI). The scale is a 100-item True/False self-report inventory of 18 personality characteristics/subscales (i.e., humorlessness, dependence, circumstantiality, personal destiny, obsessionalism, viscosity, emotionality, guilt, philosophical interest, anger, religiosity, hypermoralism, paranoia, sadness, hypergraphia, elation, aggression, altered sexuality) associated with TLE (5 items each) and 10 items from the original MMPI's Lie Scale that are thought to have no relationship with epilepsy. A subset of individuals in both groups also completed the Beck Depression Inventory–II (BDI–II)
17 and the Mini-Mental State Exam (MMSE
18).
Data Analysis
Group means and standard deviations (SD) were calculated for each BFI subscale, as well as an overall Total score. Group differences on demographic characteristics and BFI subscales were tested with a series of independent-sample t-tests. An alpha level of 0.05 was used for the error value; chi-square analysis was used to determine whether there were differences for endorsement frequency between the groups on the BFI subscales. Pearson correlations were calculated to examine the relationship between demographic factors, seizure characteristics, and the BFI scale.
RESULTS
Table 1 presents demographic characteristics of the groups. The groups were not significantly different on characteristics of gender, age, years of education, or performance on the MMSE. NES participants showed a higher level of depression on the BDI–II (mean endorsement in the “mild” range) than the TLE group (mean endorsement in the “minimal” range), although this difference did not reach statistical significance. TLE patients were significantly younger than NES individuals at seizure onset, and TLE patients had much longer illness duration than the NES group. There were more women in the NES group then the TLE group. The NES group reported greater seizure frequency then the TLE group, but the difference did not reach statistical significance. Total BFI scores for individuals with TLE (mean: 30.54; SD: 4.84) did not significantly differ from individuals with NES (mean: 33.03; SD: 15.84; t[76]=0.72; NS.)
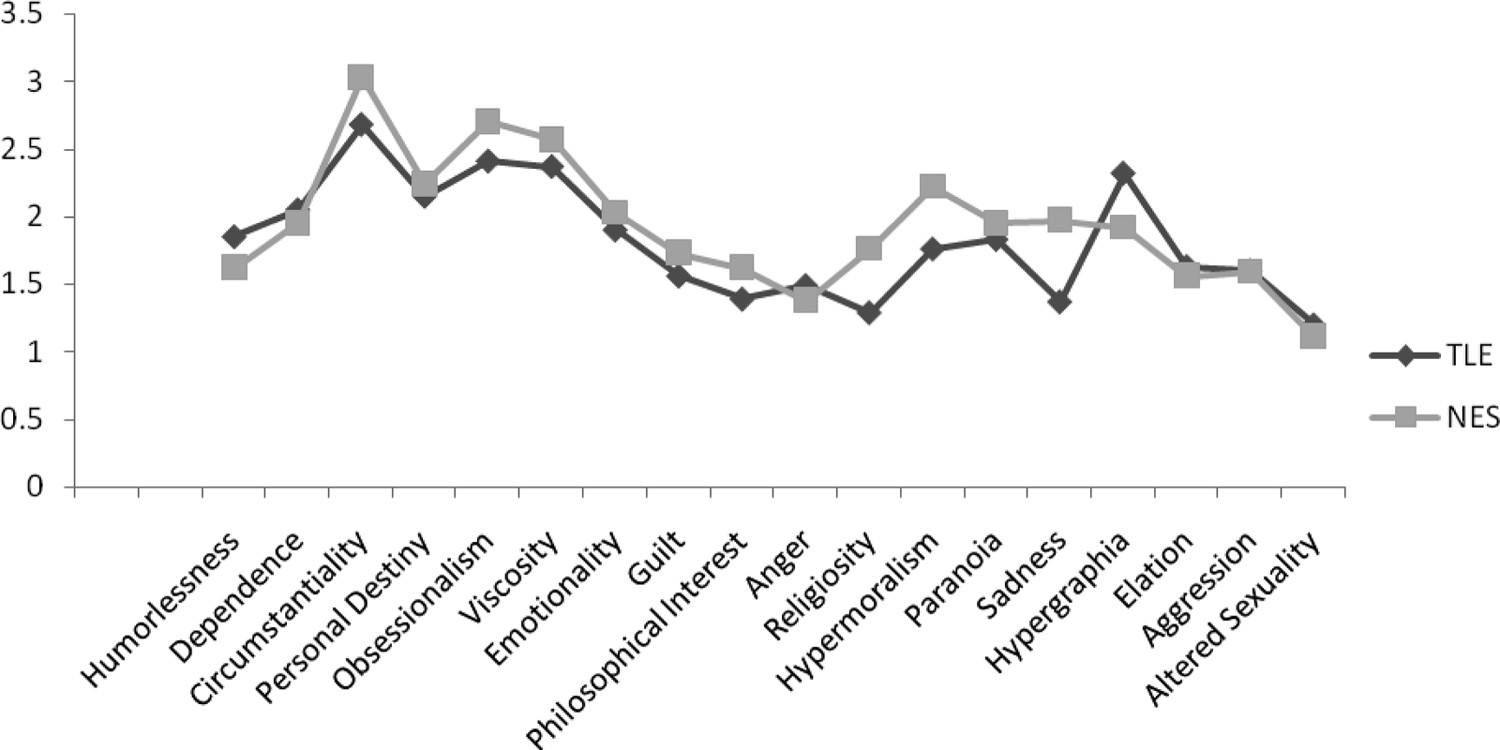
Figure 1 presents the average BFI subscale scores for each group. There were no significant differences between the groups on any of the subscales. The only trend in the data was for the Sadness subscale, with the NES group reporting higher values than the TLE group. We calculated the percentage of patients in each group who endorsed at least one trait in a behavioral domain (
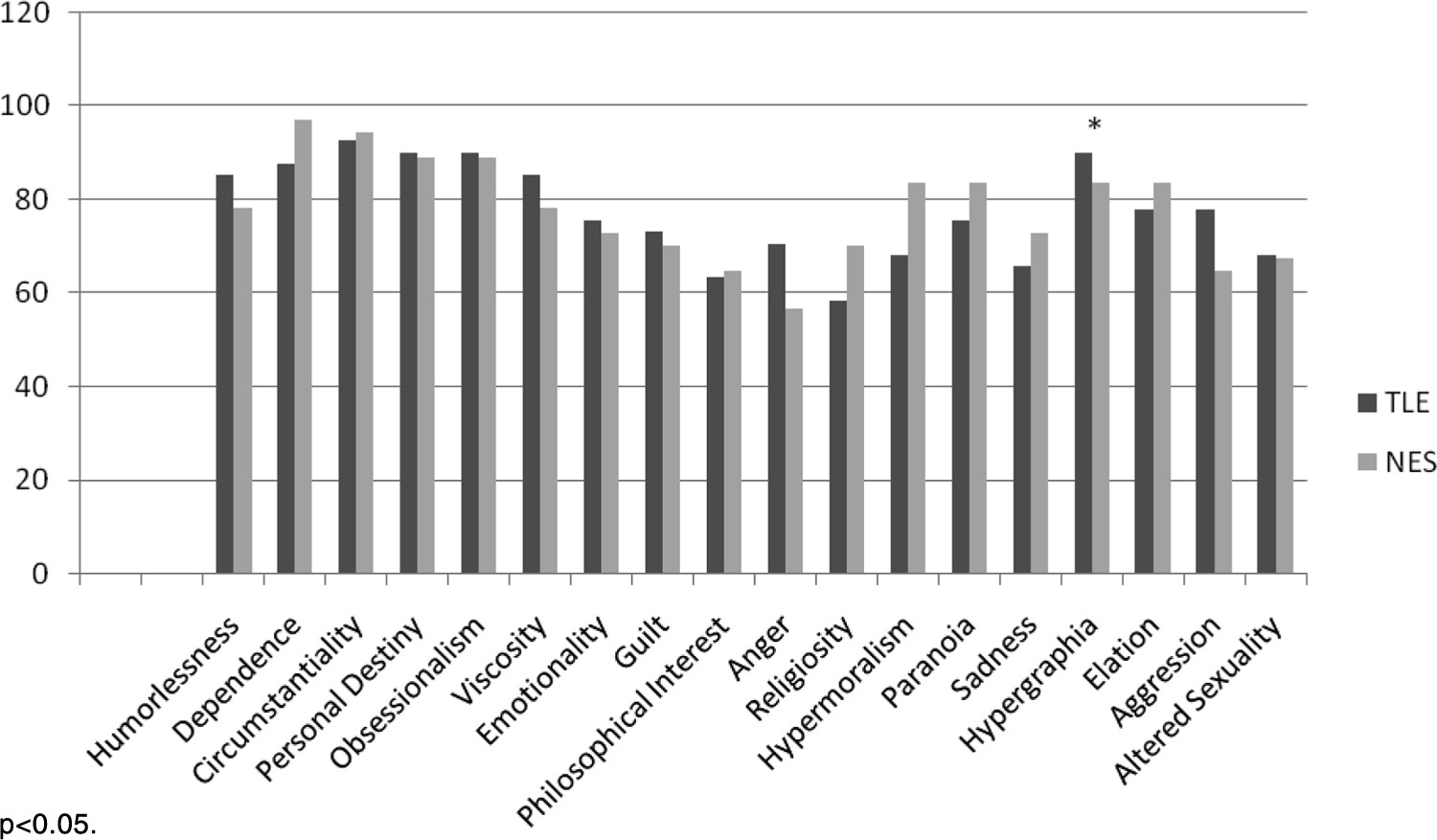
Figure 2). Again, most of the subscales did not statistically differ between the groups, although we found a significantly higher percentage of endorsement of hypergraphia in individuals with TLE, as compared with those with NES. Finally, we compared TLE and NES groups on the seven items found to discriminate best between individuals with epilepsy and controls in the original Bear and Fedio paper. These items were drawn from subscales measuring Belief in Personal Destiny, Humorlessness, Hypergraphia, Obsessionalism, Circumstantiality, and Dependence. On average, TLE patients endorsed 2.85 (SD: 1.39) items, and NES patients endorsed 2.49 (SD: 1.26) items, but this difference was not significant.
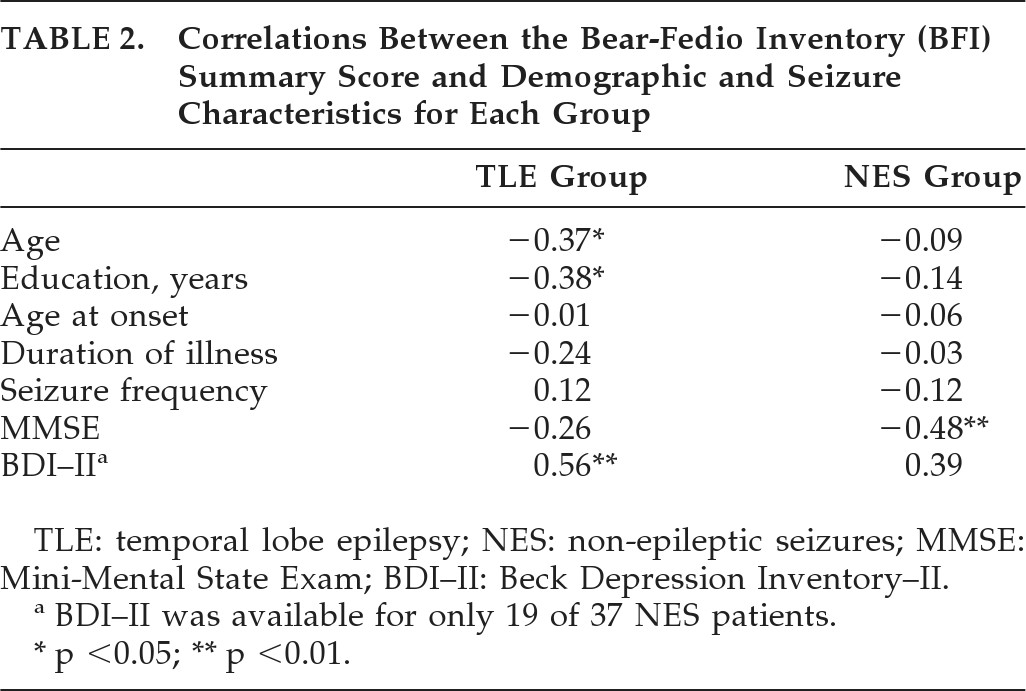
Table 2 presents correlations between demographic and seizure characteristics and the BFI Total scores by group conducted on a subset of participants. For NES patients, the BFI Total score significantly correlated with MMSE, suggesting that low scores on the MMSE were associated with higher BFI scores. There was no relationship between the BFI and age, years of education, age at seizure onset, or duration of illness. For the TLE group, higher BFI Total scores were associated with younger age. Higher BFI total scores were also related to lower levels of education among TLE patients. Finally, higher BFI scores were associated with higher self-reported depression on the BDI–II.
DISCUSSION
Results of the current study indicate that patients with NES and TLE endorse a similar number and pattern of personality characteristics. There was only a trend for NES patients to endorse greater feelings of sadness than TLE patients. These findings are consistent with evidence suggesting a very high prevalence of depression and other mood disorders in NES.
1 We found that TLE patients were more likely to endorse at least one statement related to hypergraphia than individuals with NES. Hypergraphia is one of the original characteristics identified by Geshwind as part of the TLE behavioral syndrome.
8 Our findings are consistent with those in the cognitive testing literature, in that patients with TLE or NES are difficult to distinguish by neuropsychological testing.
19 Results suggest that evaluation of personality factors such as those measured by the BFI may not be an effective additional strategy for differentiating true epilepsy from NES. Our findings underscore the similarities between patients with epilepsy and those with NES. Given the lack of personality differences between TLE and NES patients, the current findings argue against a specific temporal lobe epilepsy interictal personality syndrome. Previous research has found that TLE and psychiatric patients endorse similar levels of BFI symptoms.
15 Patients with NES meet diagnostic criteria for a somatoform disorder, among other Axis I and II conditions. Bear and Fedio proposed that the TLE personality changes might be due to a hyperconnection in the limbic system that leads to a “suffusion of experience with emotional coloration.”
11In contrast, our findings suggest that the personality changes seen in both groups may be related to the experience of having seizures, rather than specific pathophysiologic derangements in the limbic system. It is possible that the unpredictable nature of seizures, the altered state of consciousness during events, unexplained experiences and symptoms, and reactions by family member and others to seizures contribute to these personality characteristics. Some past studies have shown that individuals with generalized epilepsy endorse BFI symptoms at levels comparable to individuals with TLE, which provides further support for the nonspecific nature of the syndrome.
20 Based on our findings, we cannot entirely rule out the possibility that the NES patients have limbic dysfunction. Interestingly, there is a published case series of three individuals with NES who had evidence of mesial-temporal sclerosis.
21 Alternatively, there may be a shared pathology not associated with a focal lesion, per se, but residing in similar networks. Supporting this idea are functional neuroimaging studies that show amygdala dysfunction in patients with somatoform disorders.
22 Moreover, the finding of reduced brain-derived neurotrophic factor in patients with epilepsy or NES may be a product of similar physiologic responses to seizures between the two patient populations.
23 Neuropsychological batteries repeatedly largely fail to distinguish between the epilepsy and NES populations.
24 Other similarities between groups with epilepsy or NES in the literature include the correlation of depression and quality of life (QoL) and the lack of correlation between seizure frequency and QoL.
25,26 Future research should include structural and functional imaging to address these issues. Despite the broad similarities between the two groups, TLE and NES showed different patterns of correlations between BFI scores and demographic/seizure characteristics, which may provide insight into the underlying mechanisms for these behaviors.
We found that poorer cognitive functioning was associated with greater endorsement of these personality traits in the NES group, but not in TLE individuals, even though MMSE performance was comparable between the groups. This could suggest that NES patients with lower cognitive abilities have poorer coping strategies and experience more behavioral symptoms. There is evidence that greater psychopathology in NES is related to worse cognitive functioning.
27 Because our findings are correlational, we cannot determine the direction of this relationship; it might be better measured with more comprehensive cognitive testing (e.g., IQ). We also found that younger age, less education, and higher depression scores were associated with higher endorsement rates on the BFI for individuals with TLE. Few studies have examined these correlates or mediating variables of the BFI. In the original study, Bear and Fedio found that earlier age at onset in TLE patients was associated with greater personality and behavioral symptoms. They did not find any relationship between the BFI and education level. The overall level of symptom endorsement was lower in our sample of TLE patients than in those from the original study. Our sample population was about 10 years older than those of Bear and Fedio, and it may be that the intensity of these characteristics attenuates over time. Consistent with the Bear and Fedio study, our TLE sample was diverse in terms of lesions, which is representative of TLE patients, in general. A limitation of the current study is that we did not include a healthy-control group to serve as a comparison, although both TLE and NES groups endorsed more symptoms than healthy-controls in the original Bear and Fedio study. We also did not obtain an informant/observer measure as was done in some of the previous studies. There may be a reporting bias, as it may be that patients themselves are not fully aware of their personality changes or characteristics. Future studies could address this issue. A strength of the study is that NES patients had underlying epilepsy ruled out, using the current gold standard long-term monitoring procedure. Our NES sample was representative of the known demographic distribution of the NES population (80% women, later onset, and higher levels of depression than in epilepsy).
28,29 Our study adds to the growing evidence that patients with epilepsy or with NES exhibit many similarities, despite their different etiologies. This is the first published study of which we are aware to find that individuals with NES endorse personality characteristics previously associated with TLE.