Vitamin B
12 deficiency, which is often overlooked in clinical practice, is a common cause of neuropsychiatric symptoms in elderly patients. It is estimated that up to 40% of older adults have vitamin B
12 (cobalamin) deficiencies, and most are due to cobalamin malabsorption.
1 High prevalence of gastrointestinal (GI) pathology and use of medications that alter cobalamin pharmacokinetics are factors that increase the risk of deficiency among elderly patients. The disease process ranges from subclinical metabolic changes, to clinical symptoms, to irreversible structural damage, such as periventricular white-matter lesions. The relationship between vitamin B
12 deficiency and cognitive impairment has been well established, and a growing body of evidence also suggests a role of vitamin B
12 deficiency in mood and psychotic disorders. In its early stages, this readily treatable condition is often missed because of lack of awareness among clinicians; as well as their reliance on current “normal range” serum vitamin B
12-level parameter measures, which have a low sensitivity and specificity in diagnosing deficiency states.
2,3 Raising clinician awareness in order to accurately diagnose and treat vitamin B
12 deficiency early on can prevent irreversible structural damage and reduce morbidity among elderly patients.
COBALAMIN METABOLISM AND PATHOPHYSIOLOGY
Cobalamin is a family of complex molecules, consisting of a cobalt-containing tetrapyrrole ring and side nucleotide chains attached to the cobalt atom.
4 It is synthesized by anaerobic bacteria and is found in foods of animal origin (e.g., fish, meat, dairy products, and eggs), as well as fortified cereals.
5–7 The Recommended Daily Allowance (RDA) of vitamin B
12 is 2.4 μg per day (mcg/day) for persons over the age of 14 years. In the United States, the average daily adult dietary intake of vitamin B
12 is about 5 mcg–30 mcg, of which only 1 mcg–5 mcg are effectively absorbed, given its complex absorption process. It is estimated that only 50% of dietary vitamin B
12 is absorbed by healthy adults.
7 Defects at any step of the absorption process can cause cobalamin deficiencies of varying degrees; 50%–90% of cobalamin stores in the body (3 mg–5 mg) are located in the liver. These stores help delay, often for up to 5 years, the onset of clinical symptoms due to insufficient cobalamin absorption.
Dietary cobalamin is bound to animal proteins. In the stomach, hydrochloric acid (HCL) and pepsin are critical for the release of free cobalamin from the proteins. Glycoproteins called R-proteins (R) secreted from salivary glands and parietal cells bind free cobalamin in the stomach. Intrinsic Factor (IF), a weak binder of cobalamin in the presence of R, is also released by parietal cells in the stomach. In the duodenum, dietary- and bile-secreted cobalamin-R complexes are cleaved by pancreatic enzymes, and free cobalamin is then bound to IF with more affinity. Cobalamin–IF complexes are taken up by endocytosis, by adhering to cubilin receptors located on the distal ileal mucosa. Once inside the cell, cobalamin dissociates from IF. Free cobalamin is then bound to transporter proteins: transcobalamin (TC) I, II, and III, and transported to the liver. TC II represents about 10% of total transcobalamin and is the only cobalamin-transport protein that reaches target cell receptors. This biologically-active form of the vitamin can be taken up by cells via endocytosis for metabolic purposes.
Up to 1%–5% of free cobalamin is also absorbed throughout the intestinal mucosa, via passive diffusion.
1 This enables the absorption of high doses (at least 1 mg daily) of oral supplemental cobalamin, despite absorption-disease processes. Enterohepatic cobalamin absorption is another important source of vitamin B
12. Cobalamin released through bile is reabsorbed in the ileum on a daily basis.
8 The active forms of cobalamin (methylcobalamin and adenosylcobalamin) serve as co-factors for enzymes and exert their physiologic effects in the cytoplasm and the mitochondria. In the cytoplasm, methylcobalamin is a co-factor for methionine synthase, an enzyme necessary for two major cellular processes: 1) the conversion of homocysteine to methionine; and 2) the conversion of methyl-tetrahydrofolate (MTHF), the major circulating form of folate, to tetrahydrofolate (THF), the active form of folate, which is important for nucleotide and DNA synthesis. In the mitochondria, adenosylcobalamin catalyzes the conversion of methylmalonyl Coenzyme A (CoA) to succinyl-CoA, for lipid and protein metabolism.
6Disruptions in these pathways produce elevated levels of homocysteine (Hcy) and methylmalonic acid (MMA), respectively. Hcy is known to be neurotoxic, through overstimulation of the N-methyl-D-aspartate (NMDA) receptors, and toxic to the vasculature because of activation of the coagulation system and adverse effects on the vascular endothelium.
9 MMA, a product of methylmalonyl-CoA, can cause abnormal fatty-acid synthesis, affecting the neuronal membrane.
8 MMA and Hcy levels are elevated before any clinical manifestations of vitamin B
12 deficiency and often precede low serum vitamin B
12 levels.
5 Neuropsychiatric symptoms usually precede hematologic signs and are often the presenting manifestation of cobalamin deficiency.
10–12Vitamin B12 Deficiency
Vitamin B
12 deficiency definitions vary and usually rely on population statistics to establish normal serum-level thresholds (normal range: 180 pg/ml–900 pg/ml). This can be problematic because individual metabolic requirements may vary, and active disease can be present despite a “normal level.” False-negative results can also be explained because vitamin B
12 levels are altered by the concentration of its binding proteins, and radioimmunoassays may detect inactive forms of cobalamin that may mask tissue deficiencies of active cobalamin. Studies have found that relying on the serum levels of vitamin B
12 underestimated the prevalence of elevated metabolites that indicate tissue-deficiency by as much as 50%.
13 As deficiency develops, serum values may be maintained at the expense of tissue cobalamin. Thus, a serum-cobalamin value above the lower normal cutoff point does not necessarily indicate adequate cobalamin status. A deficiency spectrum ranging from subclinical metabolic abnormalities to clinical symptoms could be better delineated by measuring Hcy and MMA levels
2,4,14,15 or by measuring cobalamin bound to TC II (holo-transcobalamin) levels, which represent the active form of the vitamin.
16,17 A recent study in elderly persons (N=700) found holo-transcobalamin (holo-TC) to be the best predictor for determining cobalamin deficiency, when compared with other measures (serum cobalamin, Hcy, and MMA) and was recommended as the first-line measure in assessing cobalamin status,
18 but results have been inconsistent,
19 and further research is warranted.
Epidemiology
It is estimated that between 3% and 40% of older adults have vitamin B
12 deficiencies, where lower rates are seen in the community and higher rates in institutional settings.
1,8,20–22 Prevalence rates vary according to economic status, age, and dietary choices.
5,23 In a multi-ethnic study, elderly white men had higher deficiency prevalence rates than elderly black or Asian American women.
24 The elderly population is especially at risk for cobalamin deficiency, given their higher prevalence of atrophic gastritis and other GI pathology, as well as the use of medications that can interfere with B
12's absorption and/or metabolism: 10% to 30% of older people are unable to adequately absorb vitamin B
12 from foods.
23Currently, it is estimated that food-cobalamin malabsorption syndrome causes most vitamin B
12 deficiency, accounting for 60%–70% of cases, followed by pernicious anemia (PA), an autoimmune loss of secretion of intrinsic factor, which accounts for 15%–20% of cases.
1 In food-cobalamin malabsorption, there is an inability to release cobalamin from food or transport proteins, thus affecting absorption, even though unbound cobalamin can be adequately absorbed.
Etiology
Vitamin B
12 status relies not only on maintaining an adequate nutritional intake, but also ensuring an appropriate absorption process. Many different factors and conditions can interfere with this process, leading to deficiency states. The following are causes/risk factors for cobalamin deficiency:
1,25–291. Food-Cobalamin Malabsorption: atrophic gastritis (>40% in elderly persons); chronic gastritis; and drug interactions, including metformin or commonly-prescribed drugs that can increase gastric pH, such as histamine receptor-2 antagonists (H2-blockers), proton-pump inhibitors (PPI), and antacids. These drugs may also increase small-intestinal bacterial overgrowth (SIBO), which is present in 15%–50% of elderly patients, which may also increase the risk of vitamin B12 deficiency.
2. Autoimmune: pernicious anemia, Sjogren's syndrome.
3. Surgical: post-gastrectomy syndrome, ileal resection.
4. Decreased intake or malnutrition: vegetarians; chronic alcoholism; elderly people.
5. Intestinal malabsorption: chronic pancreatitis (exocrine insufficiency), Crohn's disease, Whipple's disease, celiac disease, amyloidosis, scleroderma, intestinal lymphomas or tuberculosis, tapeworm infestation, bacterial overgrowth.
6. Drugs: metformin, antacids, H2-blockers, PPIs, colchicine, cholestyramine, anticonvulsants, antibiotics, nitrous oxide.
7. Increased demands: pregnancy and lactation.
8. Genetic: transcobalamin II deficiency.
CNS Pathophysiology
Cobalamin is critical to CNS functioning and brain aging status.
30 Its deficiency can cause not only brain dysfunction, but structural damage, causing neuropsychiatric symptoms via multiple pathways. Possible mechanisms that could explain neuropsychiatric symptoms in cobalamin-deficiency states include 1) derangements in monoamine neurotransmitter production as cobalamin and folate stimulate tetrahydrobiopterin (BH4) synthesis, which is required for monoamine synthesis;
13 2) derangements in DNA synthesis; as well as 3) vasculotoxic effects and myelin lesions associated with secondary increases in Hcy and MMA levels, respectively.
31–33 Cobalamin deficiency may also indirectly cause a functional folate-deficiency state with its secondary metabolic consequences: high Hcy levels, decreased monoamine production, decreased S-adenosylmethionine (SAM) production, and abnormal methylation of phospholipids in neuronal membranes, potentially affecting ion channels and second messengers.
13In depression, disruption in methylation (one-carbon transfer) reactions in the CNS necessary for the production of monoamine neurotransmitters, phospholipids, and nucleotides
34,35 may be a mechanism contributing to pathology. Cobalamin is also required for the synthesis of SAM, which is known to have antidepressant properties.
36In cognitive impairment, a proposed underlying pathophysiologic mechanism involves cobalamin deficiency leading to hyperhomocysteinemia (HHcy), which is a risk factor for dementia, by causing 1) agonism of N-methyl-D-aspartic acid (NMDA) receptors, leading to excessive intracellular calcium influx and cell death; 2) a state of hypomethylation, leading to DNA damage and apoptosis; 3) inhibition of hippocampal neurogenesis; 4) decreased gamma-amino butyric acid (GABA)-mediated inhibitory function; and 5) blood–brain barrier (BBB) dysfunction and endothelial cell toxicity. Cobalamin deficiency has also been found to cause myelin damage by increasing myelinotoxic and decreasing myelinotrophic growth factor and cytokines.
32,37 In the absence of HHcy, however, there is less evidence to suggest that cobalamin deficiency is a risk factor for dementia.
37 Low cobalamin levels have also been reported in normal-control subjects and non-dementia patients with other neurologic diseases.
33Radiologic manifestations of low vitamin B
12 or HHcy include 1) leukoaraiosis: periventricular leukoencephalopathy or subcortical arteriosclerotic encephalopathy, manifested as white-matter hypodensity on CT scan or hyperintensity on T
2-weighted MRI; 2) brain atrophy; and 3) silent brain infarcts. These findings have also been associated with other conditions, and, in the absence of HHcy, some studies have not found an increased risk for leukoaraiosis in people with low vitamin B
12 levels.
37Neuropsychiatric Symptoms
Neuropsychiatric symptoms due to vitamin B
12 deficiency, which have been described since the early 1900s, often precede hematologic abnormalities.
10,38 Commonly-described neuropsychiatric manifestations associated with vitamin B
12 deficiency include motor, sensory, and autonomic symptoms; cognitive impairment; and mood and psychotic symptoms. The incidence of neuropsychiatric symptoms among individuals with vitamin B
12 deficiency has been reported to be 4%–50%.
33 Some of these symptoms include paresthesias, ataxia, proprioception and vibration loss, memory loss, delirium, dementia, depression, mania, hallucinations, delusions, personality change, and abnormal behavior.
10,39–42Neurologic Symptoms
Neurologic symptoms have been the hallmark of vitamin B
12 deficiency for many years, especially subacute combined degeneration (SCD) of the spinal cord in the context of pernicious anemia. In this condition, myelin in the lateral and posterior columns of the spinal cord degenerates secondary to cobalamin deficiency. It is now well known that neurologic signs and symptoms can develop before or in the absence of hematologic findings.
10,42–44 These signs and symptoms include paresthesias, ataxia, proprioception and vibration loss, abnormal reflexes, bowel/bladder incontinence, optic atrophy, orthostatic hypotension, and autonomic disturbances. Clinical neurologic manifestations can correlate with radiologic findings in the spinal cord, and reversibility has been reported with early cobalamin treatment.
45–47Psychosis
Psychosis can be the presenting symptom in vitamin B
12 deficiency.
48 The association of psychotic symptoms and cobalamin deficiency has been described for more than a century, through case reports and other studies.
10,12,13,38–40,48–50 Reported symptoms include suspiciousness, persecutory or religious delusions, auditory and visual hallucinations, tangential or incoherent speech, and disorganized thought-process.
13 A causal association has been suggested since the early 1980s, when EEG abnormalities were documented in patients with pernicious anemia.
12 Both EEG abnormalities and psychotic symptoms associated with cobalamin deficiency have shown a response to treatment with vitamin B
12, strengthening the association.
10,12,50 Another study found lower levels of vitamin B
12 in patients with psychotic versus nonpsychotic depression.
34 Some reports and studies have recognized the association of psychosis and cobalamin deficiency, specifically in older adults,
1,8,39 where, given the higher prevalence of vitamin B
12 deficiency, more cases are expected.
Mood Disorders
Depression
A growing body of literature has documented an association of vitamin B
12 deficiency and depressive symptoms in elderly patients.
51,52 These studies go well beyond case-series reports and include large-scale, cross-sectional, and prospective studies. Similar associations had been found with low folate and hyperhomocysteinemia, but a cross-sectional study of community-dwelling individuals older than age 55 with depressive symptoms (N=278) found that vitamin B
12 deficiency was independently associated with depression, whereas low folate and high homocysteine levels were associated with cardiovascular disease and physical comorbidity.
51 In this study, causality was debated because it could not be demonstrated whether low vitamin B
12 levels preceded depression or were a result of it, even though no relationship was found with self-reported decreased appetite and low vitamin B
12 levels. These results were consistent with an earlier study of community-dwelling older women (N=700), which found a twofold risk of severe depression in elderly women with metabolically significant (elevated MMA levels) vitamin B
12 deficiency.
52 A community-based, cross-sectional study in Chinese elderly persons (N=669), reported that vitamin B
12 deficiency (<180 pmol/liter) was significantly associated with depressive symptoms (odds ratio [OR]: 2.68), independent of folate and homocysteine levels.
53 Another recent cross-sectional and prospective study, in Korean people older than age 65, without depression (N=521) found that lower baseline vitamin B
12 and folate levels, and raised Hcy levels, were risk factors that predicted onset of late-life depression.
54 These findings suggest an important association between vitamin B
12 levels and depressive symptoms, supporting the approach of measurement and replacement of vitamin B
12 in the treatment of depression in clinical practice. Adequate vitamin B
12 levels may also play a role in depression treatment - response; a naturalistic prospective study (N=115) of outpatients with major depressive disorder (MDD) reported that adequate levels of vitamin B
12 correlate with a better response in the treatment of depression.
55There is currently no recommendation to use vitamin B
12 prophylaxis for depression, and a randomized placebo-controlled trial in elderly men did not find a difference in reducing the severity or incidence of depressive symptoms over 2 years when using vitamins B
12, B
6, and folate.
56 Nevertheless, we recommend ensuring adequate vitamin B
12 levels and replacing deficient levels in order to improve treatment response.
Mania
Symptoms of mania have been described in the presence of vitamin B
12 deficiency for decades,
39 even though very few case reports have been published in subjects without other comorbidities that could contribute to such symptoms.
57–60 Given the pathophysiologic mechanisms described above leading to white-matter lesions and the known association of white-matter lesions with bipolar disorder,
61 we believe it is very likely that manic symptoms can be associated with vitamin B
12 deficiency. We recognize that there is a need for further research to better understand this association and possible mechanisms, yet we recommend screening and supplementing vitamin B
12 when appropriate in the presence of mania, especially when there is no psychiatric or family history of bipolar disorder.
Cognitive Impairment
Low serum vitamin B
12 levels have been correlated negatively with cognitive functioning in healthy elderly subjects.
62,63 The association of vitamin B
12 deficiency and cognitive dysfunction has been extensively documented,
25,33,64–66 and some authors state that it can be linked credibly to mental decline.
67 Symptoms described include slow mentation, memory impairment, attention deficits, and dementia.
25,33,41 It has been suggested that low vitamin B
12 levels may cause a reversible dementia that can be differentiated from Alzheimer's disease through neuropsychological evaluation,
68 but other authors argue that there is insufficient evidence to support a specific profile of cognitive impairment associated with vitamin B
12 deficiency
69 and that dementia of the Alzheimer type is a compatible diagnosis.
37 In patients with Alzheimer's disease, low vitamin B
12 level has been associated with greater cognitive impairment.
70When considering dementia related to vitamin B
12 deficiency, an important challenge has been addressing causality, because a decline in functioning and changes in nutrition associated with dementia can cause vitamin B
12 deficiency. A previous association has not been consistently documented, as some cohort studies have shown that low vitamin B
12 level increases the risk for cognitive impairment or dementia,
65,71–76 whereas other studies have not demonstrated an increased risk.
37,76–84 The evidence is more consistent when HHcy is present, and vitamin B
12 deficiency can lead to HHcy, a risk factor for cognitive impairment and dementia.
37The reversibility of this dementia syndrome has also been questioned, given that studies reviewing large series of cases or decades of literature have yielded one and three cases of vitamin B
12 reversibility, respectively.
39,85 The evidence for response to treatment is better when pernicious anemia has been identified as the cause of vitamin B
12 deficiency and it has been treated early in the course of the disease, before irreversible damage occurs.
37 We acknowledge that the severity and chronicity of symptoms, as well as comorbid conditions and adequacy of treatment, are all important factors affecting response and reversal of symptoms.
Current guidelines suggest assessing vitamin B12 levels in patients with cognitive impairment, or as part of a workup for dementia. We believe this remains a sound clinical judgment until newer evidence can clarify the issue, as vitamin B12 deficiency can lead to HHcy, a known risk factor for dementia. If vitamin B12 deficiency is diagnosed and treated early in the course of the disease, neuropsychiatric symptoms may be prevented or even reversed.
Delirium
The hallmark of delirium remains a fluctuating level of consciousness, with attention deficits. Vitamin B
12 deficiency has been associated with attention deficits, acute mental-status changes, and acute cognitive changes, with EEG abnormalities.
13,86 Case reports describe associations of vitamin B
12 deficiency and delirium with or without other risk factors such as dementia and infection.
87,88 In a prospective study of patients with mild-to-moderate dementia with low vitamin B
12 levels that were supplemented, delirium risk was reduced significantly; however, no long-term improvement was seen in cognition or behavioral problems.
89Diagnosis
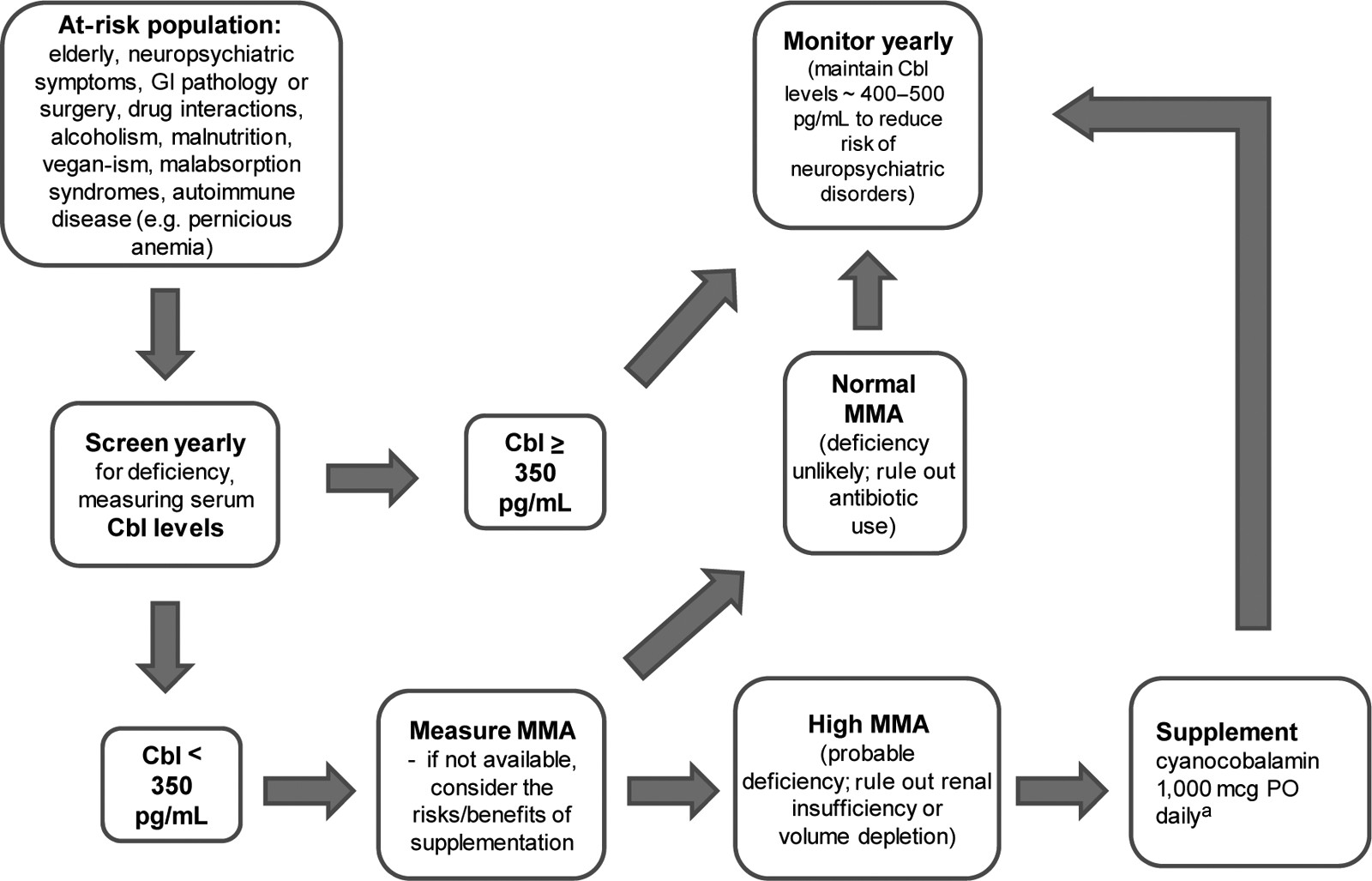
Screening for vitamin B12 deficiency should start by developing a clinical awareness of the population at risk. These include elderly persons, vegans, alcoholics, malnourished persons, and patients with GI pathology, neuropsychiatric symptoms, or autoimmune diseases. Common suggestive laboratory findings include macrocytosis with or without anemia and hypersegmented neutrophils. Special attention should also be given to patients on medications such as PPIs, H2-receptor antagonists, antacids, metformin, colchicine, cholestyramine, and patients chronically on anticonvulsants or antibiotics.
Serum cobalamin levels are unreliable when assessing vitamin B
12 status, and there has been a lack of scientific consensus defining cutoff values to diagnose deficiency states.
3,90 However, until better diagnostic tools are available, initial screening should start with assessing a serum cobalamin level. An adequate supply is suggested by levels above 350 pg/ml.
22,29 We recommend assessment of MMA in elderly patients when cobalamin levels are below 350 pg/ml.
20,26 If MMA levels are elevated, rule out other possible causes of elevated MMA, including renal insufficiency or intravascular volume depletion.
25 Patients taking antibiotics may have low levels of MMA despite vitamin B
12 deficiency, as propionic acid, a precursor of MMA,
14,25 is generated by the anaerobic gut flora, which are depleted by the chronic use of antibiotics. HHcy can be more sensitive to cobalamin deficiency, but it can also reflect folate deficiency, whereas elevated MMA has a similar sensitivity, but more specificity to metabolic vitamin B
12 deficiency.
15 Assessment of holo-TC, the active fraction of cobalamin, may also provide reliable information to evaluate vitamin B
12 status,
18 but further research is warranted. Vitamin B
12 deficiency is suspected when serum cobalamin levels are low (<350 pg/ml) and when both MMA and Hcy are elevated, or when MMA is elevated in the absence of renal disease or volume depletion, or when Hcy is elevated in the absence of folate deficiency. Several conditions can falsely elevate or decrease serum cobalamin levels, but a normal MMA and Hcy level suggest the absence of vitamin B
12 deficiency.
2 However, clinical judgment is warranted, as it has been reported that some patients may improve clinically when supplemented with vitamin B
12, despite normal levels of vitamin B
12, Hcy, and MMA, especially when PA is present.
91 When PA is suspected, or if patients fail to respond to oral, transnasal, or buccal cobalamin preparations, antiparietal cell and anti-intrinsic factor antibodies should be tested. Alternatively, if the cost of assessing MMA levels (e.g., availability, financial, time) exceeds the diagnostic benefit, we recommend doing a risk/benefit analysis to consider supplementing vitamin B
12, without further testing, in patients where deficiency is suspected, and serum cobalamin levels are less than 350 pg/ml.
Treatment
Efficacy in the treatment of vitamin B
12 deficiency due to food-cobalamin malabsorption with both parenteral and oral routes has been demonstrated. A systematic review of randomized, controlled trials of oral (PO) versus intramuscular (IM) vitamin B
12 for the treatment of cobalamin deficiency found adequate efficacy with both routes of administration;
92 PO supplementation is usually more cost-effective and convenient, and is therefore the preferred route of initial therapy. The recommended doses for PO administration vary from 125 mcg/day–1,000 mcg/day of crystalline cyanocobalamin to 1,000 mcg/day–2,000 mcg/day,
28,93,94 with a mean dose of 1,000 mcg/day being common practice. It is reasonable to initiate therapy with vitamin B
12 or a multivitamin or B-complex supplement containing at least 1,000 mcg of cobalamin daily. Transnasal and buccal cobalamin preparations are also available.
The IM route of replacement should be initiated in cases where PO, transnasal, or buccal preparations are ineffective or compliance is limited. The parenteral treatment for most cases of vitamin B
12 deficiency, where a dietary deficiency is not implicated, involves IM administration of cyanocobalamin in doses of 100 mcg/month–1,000 mcg/month, indefinitely.
8,28,95,96 An alternate scheme recommends administering 1,000 mcg/day IM for 1 week, then weekly for 1 month, and then monthly thereafter.
28 Once treatment has been initiated, obtain repeat plasma vitamin B
12, MMA, and Hcy levels in 4-to-6 weeks to assess response to treatment. Once stable mid-normal cobalamin levels are achieved, monitoring of vitamin B
12 levels should be performed every 6-to-12 months. Even though supplementation with vitamin B
12 has been proven safe, hypokalemia has been reported when treating patients with severe anemia.
33,64Prognosis
Evidence for the improvement or reversal of neuropsychiatric symptoms varies according to symptom severity, duration, and clinical diagnosis. It has been proposed that treating deficiencies in the early stages yields better results, as structural and irreversible changes in the brain may occur if left untreated. Vitamin B
12 status has been associated with severity of white-matter lesions, especially periventricular in some,
97 but not all studies.
98 The partial reversal of white-matter lesions has been documented with cobalamin treatment,
32,91 emphasizing the importance of early detection and treatment of vitamin B
12 deficiency. A correlation of vitamin B
12 treatment and decreases in MMA and total Hcy has been shown,
10 suggesting a reversal of metabolic abnormalities. Some evidence suggests that EEG, visual and somatosensory evoked potentials, and P300 latency abnormalities readily improve with treatment even if no clinical benefits are observed.
13,33Summary
Vitamin B12 deficiency is a common and often missed problem in geriatric patients. Neuropsychiatric manifestations can be the presenting and only sign of this deficiency even in the absence of hematologic abnormalities. Vitamin B12 deficiency can occur despite “normal” serum cobalamin levels; therefore, measuring Hcy and MMA can decrease false-negative findings. Early detection and treatment are important to prevent structural and irreversible damage leading to treatment-resistant symptoms. Oral treatment can be as efficacious as parenteral treatment even in the presence of pernicious anemia. Because the neurologic damage caused by cobalamin deficiency is often irreversible, and progression of disease can be abated by cobalamin replacement, it is important to maintain plasma cobalamin levels in the mid-normal range among elderly persons.
Recommendations
1. Screen annually for vitamin B12 deficiency in at-risk patients by measuring serum cobalamin levels.
2. Measure MMA levels in patients with serum cobalamin levels <350 pg/ml. High levels, in the absence of renal insufficiency or volume depletion, are suggestive of vitamin B12 deficiency.
3. If there is no access to MMA or the cost outweighs the diagnostic benefit, a clinical approach can be to supplement after a risk/benefit analysis and monitor for response when low serum cobalamin levels are present (<350 pg/ml) in the context of suggestive clinical findings.
4. Administer cyanocobalamin 1,000 mcg PO daily, even if pernicious anemia has been identified. An alternative parenteral treatment includes cyanocobalamin 1,000 mcg IM daily for 1 week, then weekly for 1 month, and then monthly thereafter.
5. Evaluate for pernicious anemia by requesting antiparietal cell and anti-intrinsic factor antibodies in patients with clinical symptoms of subacute combined degeneration of the spinal cord and suggestive hematological manifestations (e.g., macrocytic anemia). Patients with pernicious anemia require lifetime cobalamin supplementation.
6. Monitor vitamin B12 serum levels at least yearly in patients who have stopped supplementation after symptoms have improved or cobalamin levels have been replenished.
7. Maintain plasma vitamin B12 levels in the mid-normal range (400 pg/ml–500 pg/ml) to reduce risk of developing vitamin B12-related neuropsychiatric disorders.