Since 1978, disruption of prepulse inhibition of the startle response (PPI) has been widely identified in subjects with schizophrenia.
1 Startle reflex consists of a contraction of the skeletal and facial muscles in response to sudden intense stimulation. This reflex is diminished when preceded by a weak prestimulus applied between 30 and 500 msec earlier and is known as PPI. Graham suggested that PPI reflected an automatic preattentive sensorimotor gating process that briefly protected the processing of more intense prepulse stimuli.
2 The PPI is thought to reflect the activation of the behavioral filtering processes, which are regulated by the forebrain neural circuit, and it is detected in numerous species ranging from mice to humans. Deficits of PPI manifest in the inability to filter out unnecessary information, and they have been linked to abnormalities of sensorimotor gating. Such deficits are noted in patients with mental illnesses such as schizophrenia or Alzheimer's disease and in people under the influence of drugs, surgical manipulations, or mutations.
3 Deficits in sensorimotor gating are correlated with cognitive impairments, such as information processing, related to attention.
4 Bitsios and Giakoumaki also found significant correlations between PPI and some executive functions, such as planning, selective attention, and performance on the Stroop task.
5 Other studies that focused on patients with schizophrenia also reported significant correlations between PPI and lateralized attention
6 and reasoning.
7 Patients with schizophrenia show a decrease in PPI at intervals between 30, 60, and 120 msec, which reflects impairments in the filtering capacity.
8,9 It has been hypothesized that patients with schizophrenia present impairment of the preattentive filtering, which would lead to an information overload and to cognitive deficit. Deficits in sensorimotor gating are a common psychophysiological factor within schizophrenia that could involve a wide variety of impairments related to perception, attention, or reasoning.
10 Furthermore, a decrease in PPI may be considered an endophenotypic marker for schizophrenia,
7,9 as PPI has been found to be heritable and stable with repeated testing.
11 The PPI has been proposed as a stable biological marker for nonpsychiatric populations
12 and for clinical populations of patients with schizophrenia.
13 There is a wealth of opportunity to further the understanding of the etiology, course, and treatment of schizophrenia by focusing more attention on the study of patients experiencing their first episode of psychosis. This sample offers a unique opportunity to study more completely the nature of schizophrenia by examining subjects before extended neuroleptic treatment and the development of chronic symptoms.
14 Regarding PPI performance in patients with first episode psychosis (FEP), there are some findings indicating that PPI deficits are already present at the earliest stages of the clinical onset of schizophrenia, before the patients have received any antipsychotic treatment,
15 in drug-naïve first-episode patients,
16 and even before the onset of psychosis.
17 However, there is still controversy regarding the existence of PPI deficits in drug-naïve first episode patients, and some studies failed to obtain these impairments in groups of never-medicated first episode patients.
18 Some of the reasons for this controversy between studies can be found in the existence of some interfering variables, such as sex,
19 tobacco consumption,
20,21 and antipsychotic medication.
9 Therefore, these and other factors that are frequently observed in schizophrenia should be considered when comparing PPI deficits in patients with schizophrenia.
Patients with schizophrenia often report cannabis abuse,
22 and this incidence of cannabis abuse is more commonly found in these patients than in the normal population.
23,24 Rates of lifetime cannabis use have been found to range between 45% and 64.4% in schizophrenia.
25 Because these patients show significant prevalence of cannabis abuse, it is important to study its effects in this group of patients. Several hypotheses have been put forward to explain an earlier age at onset of schizophrenia in patients with cannabis abuse.
26 A possible explanation postulates that cannabis abuse and schizophrenia share common etiologic factors such as genetic vulnerability
27 or developmental neuropathology.
28 There is also accumulating evidence suggesting that cannabis use is a risk factor for the development of psychosis or schizophrenia.
29,30 Moreover, there is epidemiological evidence suggesting that cannabis use might be a risk factor for schizophrenia, and an exacerbation of the symptoms and worsening of the prognosis may occur in subjects with a predisposition to schizophrenia.
31The PPI is also impaired in subjects with a history of cannabis abuse. In a study by Kedzior and Martin-Iverson regarding active PPI paradigms, they aimed to determine whether healthy subjects abusing cannabis exhibited attention-modulated deficit in PPI. They found that this group showed significant PPI reduction compared with noncannabis abusers and that this decrease correlated with the duration of cannabis abuse.
32 These previous results were confirmed by subsequent studies.
33 Further research has been performed within animal models to study the effects of some cannabinoid components on PPI. Several studies have reported PPI impairments in rats due to the effects of cannabinoid receptor agonists.
34–36 Some data suggest that chronic stimulation of the cannabinoid receptor in rodents leads to persistent PPI disruption. Therefore, PPI reduction observed in those rats stimulated with cannabinoid agonists may be a valid animal model of sensorimotor gating deficits in patients with schizophrenia.
36The current study aimed to examine PPI of the startle reflex differences in patients with FEP in terms of the history of cannabis abuse. There is little research on the effects of cannabis use and PPI functioning in schizophrenia. As PPI impairment in patients with schizophrenia is considered to be an endophenotype for this mental disorder, and cannabis is the most common drug in schizophrenia after nicotine and alcohol,
37 it might be of great interest to study these differences. In a previous study from our group, we found that patients with schizophrenia and multiple substance abuse performed significantly worse than patients with schizophrenia and no history of substance abuse.
38 According to these results, we hypothesized that patients with FEP and cannabis abuse would register worse performance in PPI than patients with FEP and noncannabis abuse.
Methods
Participants
We used a sample of 35 patients diagnosed with FEP with schizophrenia and a control group of 22 healthy subjects. All patients consisted of subjects with their first psychotic episode, diagnosed using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I),
39 and it was the first time they received a diagnosis of psychosis. Patients were recruited from the Psychiatry Service of the 12 de Octubre Hospital in Madrid. Patients with a history of brain damage, organic diseases of the central nervous system, or mental retardation were excluded. All patients in the study had been taking antipsychotic medication during the first month prior to PPI evaluation, and all of them were receiving atypical antipsychotics. Psychotic symptomatology was assessed with the Positive and Negative Syndrome Scale.
40 Prior to PPI assessment, the average period of psychotic symptomatology lasted approximately 6 months. Control subjects were psychiatrically, medically, and neurologically healthy volunteers who were not receiving any psychiatric medication and had no first- or second-degree relatives with psychosis. All of them were recruited from the community in the same city. All patients and control subjects included in the study were older than 18 years of age.
The group of patients with schizophrenia was divided in terms of their history of cannabis abuse. We obtained a subgroup of 21 patients with FEP and cannabis abuse disorder (CUD) and a subgroup of 14 patients with FEP and no history of cannabis abuse (non-CUD). Cannabis abusers met DSM-IV criteria. The number of days since last cannabis use was estimated to be between 15 and 30 days, with an average abuse of six cannabis joints per day. Tobacco consumption was allowed ad libitum before testing to ensure that PPI was not influenced by smoking withdrawal.
20,41 Clinical and demographic characteristics are presented in
Table 1.
The study was accepted by the ethics commission of the 12 de Octubre Hospital. All subjects signed an informed consent form before starting the study.
Procedures
A commercial human startle response monitoring system was used to generate and deliver the startle stimuli and to record and score electromyographic (EMG) activity (using STARTLEH software, a WNS 220 electronic module, an ADInstrument Bio Amp, and a PowerLab data unit). Prior to PPI testing, audiometric screening excluded individuals with a hearing impairment in both the patient and control groups [threshold N40 dB(A) at 1000 Hz]. Four types of startle stimuli were presented to subjects binaurally through headphones: a pulse-alone stimulus of 100-dB white noise presented for 40 msec, and three prepulse 30-msec stimuli of 30-dB white noise presented at 30, 60, and 120 msec before the pulse. All the stimuli were presented against a continuous background noise of 55 dB. EMG recordings were taken with subjects sitting comfortably in a moderately lit soundproof room. The recording lasted 15 minutes, and patients were told to be relaxed and not to pay attention to the noises. The eye-blink component of the startle response was measured by recording EMG activity of the orbicularis oculi muscle directly beneath the right eye using two miniature silver/silver chloride disk electrodes. The ground electrode was placed on the forehead. Impedance level was kept below 5 kOhm. The startle system recorded EMG activity for 250 msec from the onset of the startle stimulus. EMG activity was band-pass filtered, with a 60-Hz notch filter used to eliminate 60-Hz interference. EMG data were stored off-line in the analytical program of the response monitoring system.
Patients were told that brief loud startling sounds would be delivered through the headphones and were asked to keep their eyes open during the test and to avoid moving. The session began with an acclimatization period to reduce initial reactivity and familiarize participants with the test. The four kinds of startle stimuli previously described were presented in a pseudorandom order. The experiment consisted of three blocks: 1) five pulse-alone trials; 2) 32 pulse-alone and prepulse-pulse trials with a 30-, 60-, and 120-msec prepulse-to-pulse interval; and 3) five pulse-alone trials. A total of 42 trials were conducted in each experiment.
All patients were evaluated between 14 and 21 days after being admitted at the Psychiatry Service in our hospital and were clinically stabilized when PPI assessment was performed.
The startle measure used was PPI, computed as the percentage reduction in amplitude throughout the pulse-alone trials and calculated as the difference of the average startle response magnitude in pulse-alone trials minus the magnitude of the average startle response in prepulse trials divided by the magnitude in the pulse-alone trials [%PPI = (pulse – prepulse)/pulse × 100].
Statistical Analysis
The SPSS statistical package version 15 was used for statistical analysis. Continuous sociodemographic variables were evaluated using one-way analysis of variance (ANOVA) and categorical variables using the chi-square test.
We used a two-way ANOVA repeated-measures design, incorporating group × interval, with PPI intervals as a within-subject factor (PPI 30%, PPI 60%, and PPI 120%), group (CUD, non-CUD, and control subjects) as a between-subject factor, and age as a covariable to control the potential effect of this variable. Significant main and interaction effects were further analyzed by post hoc comparisons with Bonferroni-adjusted alpha level.
Results
As shown in
Table 1, we only found statistically significant differences between patients and controls in the years of education (p<0.001). This result may be due to the fact that patients attending our hospital come from a low socio-cultural level.
Regarding the group of patients with FEP (CUD and non-CUD), patients with CUD were younger than patients without CUD (p=0.021), a significantly higher number of men were found within this CUD subgroup (p=0.04), and there were more patients with tobacco consumption in the CUD group compared with the non-CUD group (p<0.001). Finally, patients with CUD obtained similar scores to patients without CUD in the Positive and Negative Syndrome Scale.
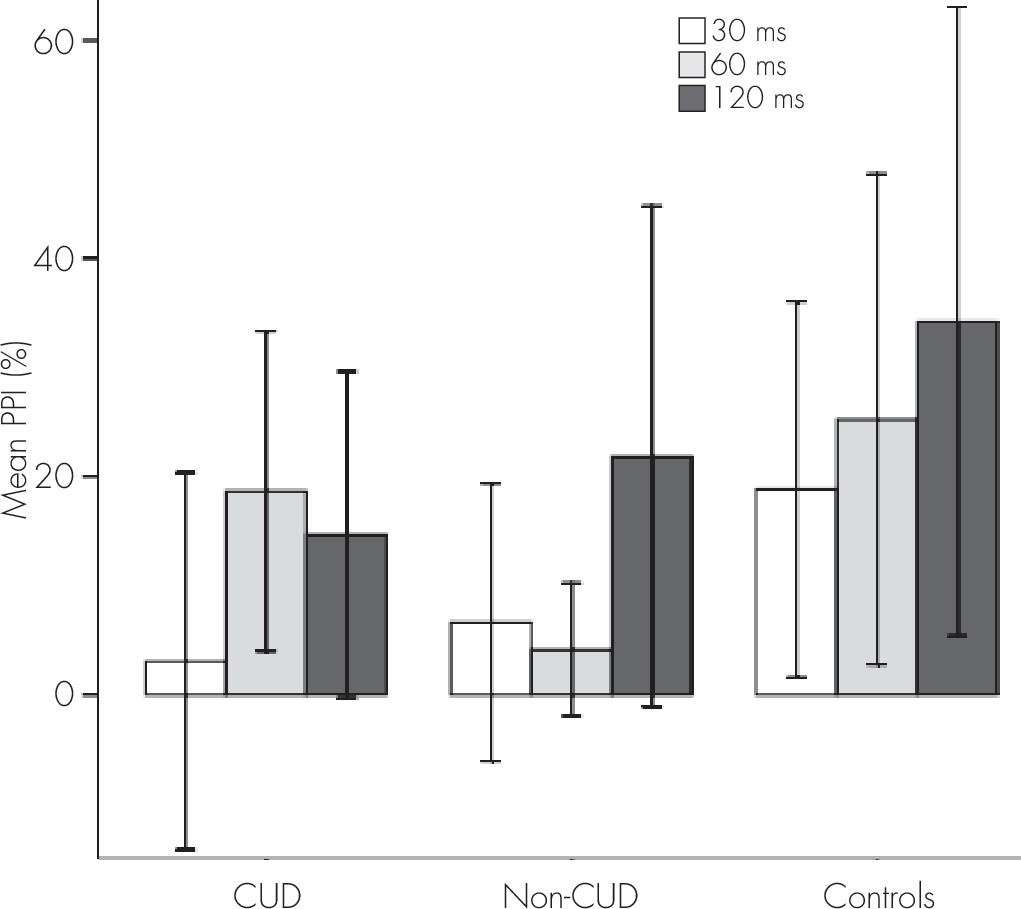
Regarding PPI measures, we found a significant main effect for group (F=24.165, df=2, 69, p=0.020). A post hoc test showed statistically significant lower amplitudes in patients without CUD compared with control subjects (p=0.037). There was also a significant group × interval interaction (F=2.951, df=4, 136, p=0.022). A Bonferroni post hoc test showed the following differences in PPI between the three groups (
Figure 1): at 30 msec, both patients with and without CUD showed lower percentages of inhibition than control subjects (p=0.044, and p=0.007, respectively); and at 60 msec, patients without CUD obtained lower PPI levels than control subjects (p=0.003). Furthermore, patients with CUD exhibited a higher PPI percentage than patients without CUD (p=0.040). No significant differences were found at 120 msec. Mean differences between groups are shown in
Table 2.
Discussion
The present study was designed to determine whether PPI was more impaired in patients with CUD, FEP, and schizophrenia compared with patients without CUD wh had FEP with schizophrenia and with a control group of healthy subjects. The results of this study showed that patients with FEP obtained lower PPI levels than healthy control subjects at 30 and 60 msec. These results are in agreement with the extensive research reporting that patients with schizophrenia have decreased inhibition of the eyeblink magnitude compared with healthy subjects.
42,43The most striking results to emerge from our study were that patients with CUD exhibited higher PPI percentages at 60 msec than patients without CUD. These results involved a potential better PPI in patients with CUD than in patients without CUD at some levels, and this finding has been recently reported in another study.
18 This may reflect the existence of an improvement in patients with CUD compared with patients without CUD in one biological measure that is related to information processing. The studies regarding the effects that cannabis abuse may exert over cognitive functioning in schizophrenia suggest that those patients that are also cannabis abusers show better functioning in several cognitive domains than patients without cannabis use.
22,44 However, although these data about cognitive performance might support our results regarding PPI improvement at 60 msec, caution must be applied. There is still some controversy about the existence of reliable correlations between PPI measures and some neuropsychological tasks.
5,9Regarding the other PPI measures examined in this study, we found that, at 30 msec, both patients with and without CUD with FEP showed impairment at this level compared with control subjects. These results suggest that at the most preattentional levels of sensory gating, deficits appear in all the patients; thus, this disfunction may be considered a potential psychophysiological marker for schizophrenia.
9 We did not find significant differences between groups in PPI at 120 msec. There results are contradictory to other studies that found deficits at 120 ms in schizophrenia.
45 One explanation for the failure to find deficits at this level in our group of patients could be that atypical antipsychotic medication may be improving PPI functioning at this late stage of sensory grating. There is evidence supporting that PPI is improved by atypical drugs.
18,46The results of the present study rejected our primary hypothesis, which suggested that patients with CUD would obtain worse performance in PPI than patients without CUD. There are two reasons that may explain why we did not find greater PPI impairments in patients with CUD compared with patients without CUD as we hypothesized. First, patients with schizophrenia that report cannabis abuse might comprise a subgroup of patients that display better premorbid adjustment and cognitive functioning.
47,48 There is some evidence suggesting that patients with schizophrenia that are cannabis abusers may have better social premorbid adjustment and may show superior social skills, which would improve their ability to manage the acquisition of illegal drugs.
22 The second explanation for our results suggests that despite that both subgroups of patients may be considered the same group, PPI would be improved in those patients that are also cannabis abusers, as a result of the increase of dopamine levels that is caused by cannabis at the mesolimbic and frontal areas.
34,35 Acute administration of cannabinoid agonists might increase frontal dopamine activity.
49 Therefore, sporadic abuse may improve frontal cognitive functioning in these patients,
50 whereas repeated or chronic exposure to cannabis might cause reduction of dopamine on the prefrontal cortex, and thus impairment in cognitive functioning would appear. For this study, the PPI task was carried out within the first few weeks after the patient was diagnosed, and all urine drug screenings in patients with CUD were positive at hospital admission. As tetrahydrocannabinol is stored in fat body tissues, several weeks are needed before the cannabinoids can be eliminated.
51 Tetrahydrocannabinol is elucidated to represent the psychoactive constituent of cannabis.
52 One study showed abnormal densities of the dopamine transporter in postmortem brains of patients with schizophrenia that did not show traces of tetrahydrocannabinol in their blood at autopsy, whereas this deficiency did not appear in patients with schizophrenia that had levels of tetrahydrocannabinol in the body.
53 Our group of patients with CUD were still under the effects of cannabis when the PPI evaluation was performed. Therefore, these levels of cannabinoids could be producing some modifications in the areas of the cerebral cortex that are related to inhibition, and improvement would be observed in this cognitive domain in patients with CUD. However, higher doses of cannabis, or chronic consumption of this substance, might impair cognition.
32Nevertheless, our findings are subject to some limitations. First, controls showed more years of education than patients with FEP, as the patients attending our hospital had relatively low socio-economic status. However, PPI reflects an automatic preattentive sensorimotor gating process,
2,54 and thus educational level should not influence automatic processes. A second limitation of this study is the relatively small sample size. However, even with a small number of patients, we obtained significant differences supporting the previous literature. Third, there were significantly higher rates of tobacco consumers within the CUD group than in patients without CUD. However, this is explained by the fact that, in Spain, all cannabis smokers consume tobacco, whereas there is lower probability of the incidence of tobacco abusers in a non-CUD group. Finally, all the patients of this study were under atypical antipsychotic treatment. Atypical antipsychotics have been found to improve PPI levels in schizophrenia,
46 so this might be influencing our results. However, even under the effects of these antipsychotics, patients with FEP showed lower values than control subjects.
Taken together, these results suggest that patients with FEP and with no history of cannabis abuse showed more major impairment in PPI at some levels than patients with a history of cannabis abuse. Therefore, patients without CUD will exhibit more difficulties in information processing, which could be involved in impairments in other cognitive functions, as was recently reported by our group.
38 In this study, we reported evidence suggesting that cannabis abuse might improve PPI performance at some levels in patients with schizophrenia, at least in the early stages of the disease. However, more work needs to be done to determine whether patients with schizophrenia and cannabis abuse perform better in PPI than those patients that are not cannabis abusers. Moreover, studying other PPI measures such as startle reactivity will be of interest to have an extensive knowledge of this functioning. Finally, more follow-up studies need to be done to determine whether these differences between groups are maintained over time or if chronic and repetitive cannabis abuse leads to worse PPI functioning in those patients with schizophrenia with a history of cannabis abuse.