Multiple sclerosis (MS) is one of the most common neurological diseases in young adults in which damage to myelin (demyelination) occurs due to inflammation in the central nervous system. In addition to physical symptoms, MS is well known to be associated with cognitive impairment and disorders of mood. Cognitive impairment is estimated to affect 40%−65% of patients with MS
1 and is now recognized as a prevalent and clinically relevant consequence of the degenerative process, separate from disability accumulated from relapses. The most frequently observed impairments are in information-processing speed, working memory, and episodic memory, along with verbal fluency and executive function (less frequently).
1 Similarly, disorders of mood, such as depression and anxiety, are frequently reported in MS. Lifetime prevalence rates in the MS population are reported at 35% for anxiety
2 and between 25% and 50% for depression,
3,4 which are higher than in other chronic medical/neurological illnesses and at least three times the rates in the general population.
In the general population, mood disorders can worsen cognitive function. In patients without MS, it is known that depression has a negative impact on working memory, processing speed, attention, and executive function—all domains that can be affected by MS as well.
5,6 Anxiety symptoms/disorders are often associated with deficits in episodic memory and executive function.
7,8 The effects of depression on cognitive impairment in MS have been explored; the relationship between anxiety and cognitive impairment has received much less attention. Depression in MS, not unlike the general population, has been associated with various cognitive deficits, particularly on cognitively demanding tasks that emphasize processing speed, memory, and executive function.
9 The impact of depression on cognitive performance is more likely to be present when depression is in the moderate-to-severe range in patients with MS.
10,11 Yet, the studies examining this topic are few and are often small in numbers. Studies have shown that both depression and anxiety lead to overestimations of the degree of cognitive impairment when patients with MS self-evaluate their cognitive function.
12,13 Only one study has examined the relationship between anxiety and cognitive impairment in the population with MS.
14 Thus, the goal of this retrospective chart review was to examine the effects of both depression and anxiety symptoms, the latter underinvestigated to date, on cognitive performance in patients with MS from a large cohort of patients with relapsing-remitting MS.
Methods
This retrospective chart review identified all male and female patients with relapsing-remitting (RR) MS assessed in the MS cognitive clinic for a cognitive assessment between January 2011 and December 2014. To be included, patients had to 1) be between 18 and 59 years old, inclusive; 2) have an RRMS diagnosis based on McDonald’s 2010 criteria
15; 3) have not received corticosteroids in the last 30 days; 4) achieved at least a ninth grade education; and 5) be fluent in English. Patients were not included if there was 1) a history of a major psychiatric disorder such as bipolar disorder, schizophrenia, or posttraumatic stress disorder; or 2) reported significant marijuana use (daily). In addition to these inclusion and exclusion criteria, patients had to have completed the Hospital Anxiety and Depression Scale (HADS)
16 and the Minimal Assessment of Cognitive Function in MS (MACFIMS)
17,18 because these were the main outcomes being examined. The HADS is a self-reported scale that detects both anxiety and depressive symptoms. There are 14 questions in total, scoring responses from 0 to 3 reflecting the severity of the symptoms. The two subscores, HADS-A (anxiety) and HADS-D (depression), both range from 0 to 21. The HADS has been validated in the population with MS; a cutoff value of 8 for either scale (anxiety HADS-A or depression HADS-D) was found to be both sensitive and specific in this population.
19 The MACFIMS is a comprehensive battery developed and validated to assess patients with MS for the presence of cognitive impairment.
17,18 The MACFIMS consists of seven neuropsychological tests covering five cognitive domains most commonly affected in MS. These tests are Judgment of Line Orientation,
20 a measure of visual/spatial perception; Controlled Oral Word Association Test,
21 a measure of generative verbal fluency; California Verbal Learning Test 2nd edition (CVLT2),
22 a measure of auditory/verbal episodic memory; Brief Visuospatial Memory Test–Revised (BVMTR),
23 a measure of visual/spatial memory; Paced Auditory Serial Addition Test 3.0 (PASAT),
24 a measure of speed and working memory in the auditory domain; Symbol Digit Modalities Test (SDMT),
25 a measure of processing speed; and the Delis Kaplan Executive Function System (DKEFS) Sorting Tests,
26 a measure of higher executive function. Using a previously reported control population,
17, z-scores were calculated for each test. As per the MACFIMS battery, scores on each test were classified as impaired with a z-score worse than –1.5. Data on the North American Adult Reading Test
27—a measure of premorbid intelligence—and Fatigue Severity Scale (FSS)—a measure of generalized fatigue validated for the population with MS
28—were also collected.
Statistical Analysis
Descriptive statistics were used for the demographic and disease-specific characteristics. Pearson’s correlations will be used to determine any relationship between HADS-A and HADS-D scores and raw MACFIMS component scores. Chi-square was used for categorical variables (positive or negative on HADS-A or HADS-D with MACFIMS categories). One-way ANOVA was use to compare HADS-A or HADS-D classification and each of the MACFIMS tests. All statistical analyses were performed using SPSS 22.0. Although this was an exploratory analysis, to minimize type I error due to multiple comparisons, the significance level was set at p<0.01.
Results
Of the 387 patients evaluated in the MS cognitive clinic between January 2011 and December 2014, a total of 151 patients met the inclusion and exclusion criteria. The main reasons for exclusion were assessment for a research protocol instead of a clinical assessment (N=90) and a disease course other than RR (N=65). This cohort had a mean age of 42.4±8.7 years; the vast majority consisted of women (77.5%) and Caucasians (97.4%), with a mean years of education achieved of 14.0±2.3 years (
Table 1). They were, on average, 9.7±7.0 years since diagnosis, with a median Expanded Disability Status Scale of 2.0 (range, 0.0–7.0), and 69.5% were receiving disease-modifying therapy. The mean FSS score for the cohort was 4.7±1.6 (
Table 2).
On the HADS, the mean anxiety score (HADS-A) was 8.2±4.0, with 81 (53.6%) in the affected range, whereas the mean depression score (HADS-D) was 5.8±3.9, with 52 (34.4%) in the affected range. The two scores correlated significantly with each other (r=0.574, p<0.001) and with the FSS (HADS-A: r=0.438, p<0.001; HADS-D: r=0.539, p<0.001). The HADS-A did not correlate significantly with any demographics, but the HADS-D correlated significantly with the Expanded Disability Status Scale (r=0.259, p=0.001).
The results from the MACFIMS battery are shown in
Table 3. The most commonly affected test was the SDMT (71, 47.0%), followed by measures of visuospatial memory (BVMTR–Immediate Recall: 53, 35.1%; BVMTR–Delayed Recall: 59, 39.1%).
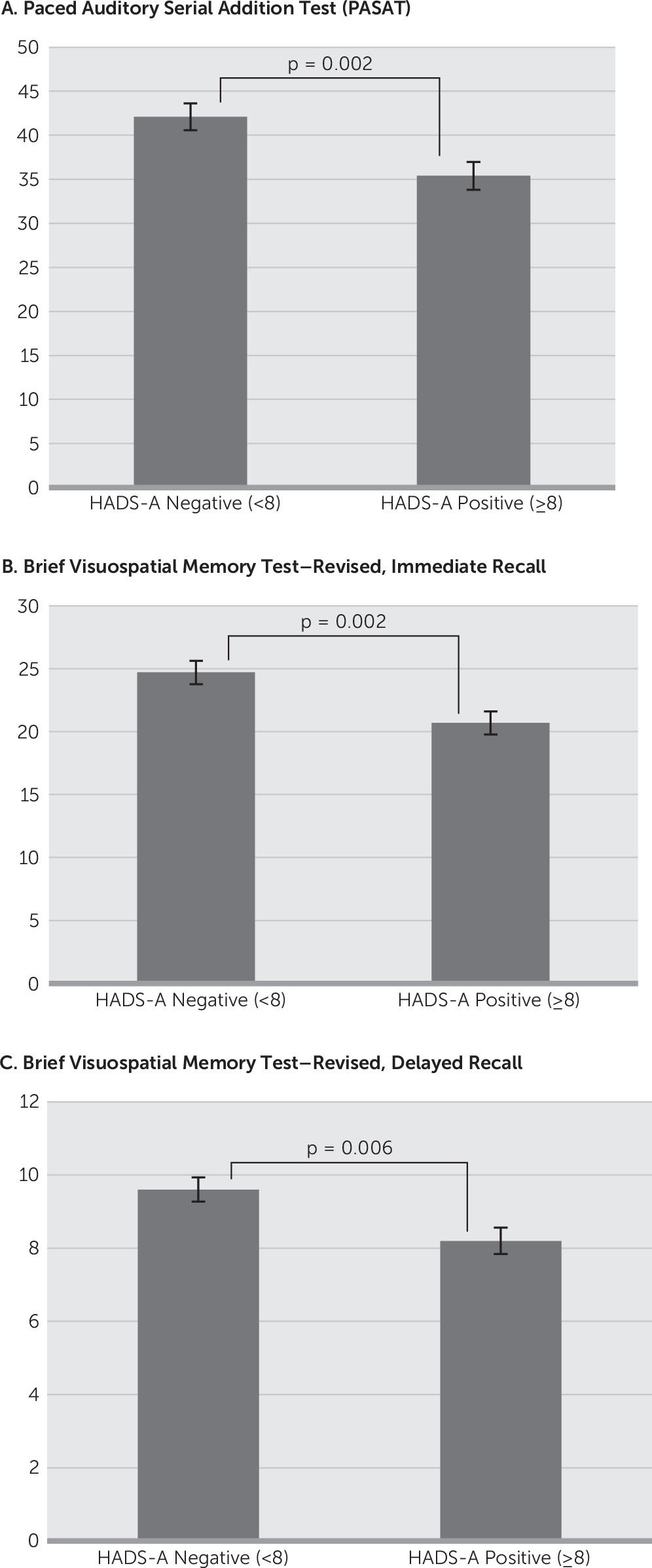
The HADS-A significantly but negatively correlated with the PASAT (r=−0.247, p=0.001), the BVMTR–Immediate Recall (r=−0.252, p=0.002), and the BVMTR–Delayed Recall (r=−0.258, p=0.002). The HADS-D score significantly but negatively correlated with the SDMT (r=−0.285, p<0.001) and the BVMTR–Delayed Recall (r=−0.236, p=0.004). Comparing the scores on these cognitive domains based on classification of normal versus affected on the HADS-A found a significant relationship with the PASAT (HADS-A<8: 42.1 versus HADS-A≥8: 35.4, p=0.002), the BVMTR–Immediate Recall (HADS-A<8: 24.7 versus HADS-A≥8: 20.7, p=0.002), and the BVMTR–Delayed Recall (HADS-A<8: 9.6 versus HADS-A≥8: 8.2, p=0.006) (
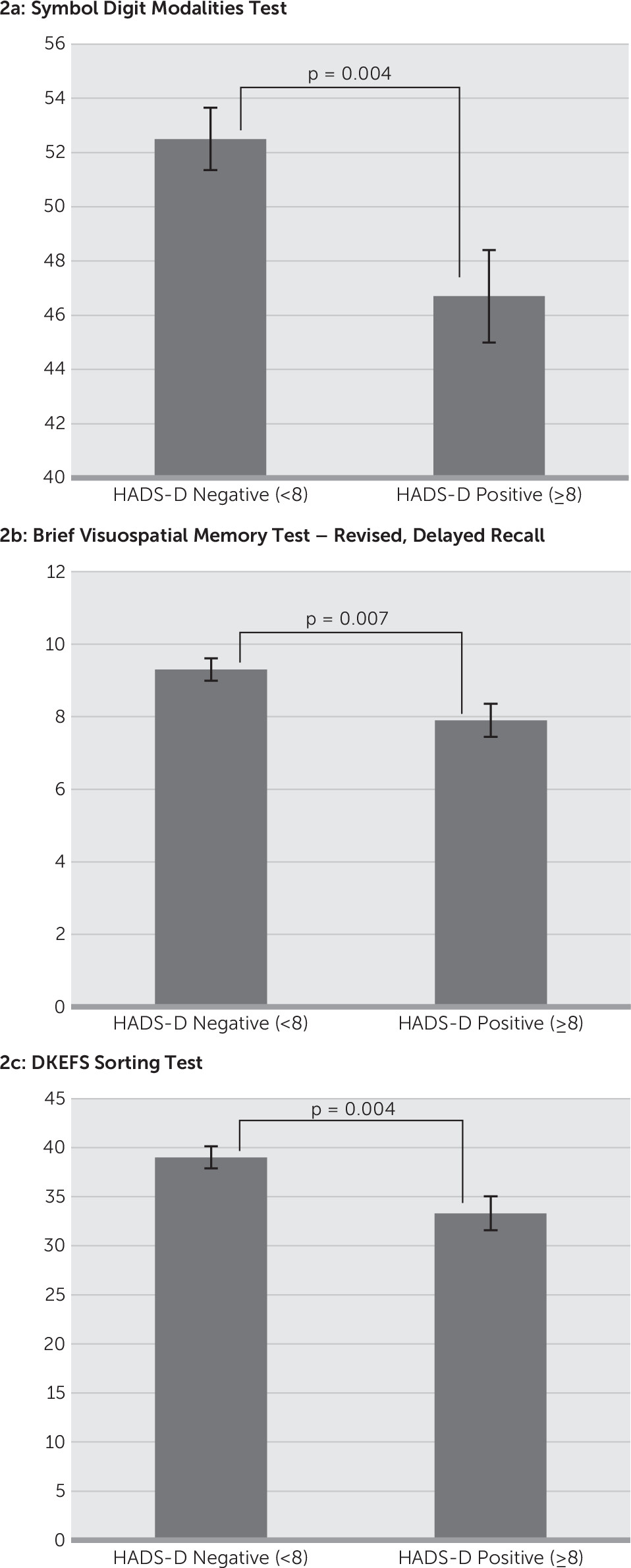
Figure 1). In contrast, comparison of the cognitive tests on the basis of classification of normal versus affected on the HADS-D found the SDMT (HADS-D<8: 52.5 versus HADS-A≥8: 46.7, p=0.004), BVMTR–Delayed Recall (HADS-D<8: 9.3 versus HADS-A≥8: 7.9, p=0.007), and DKEFS–Description (HADS-D<8: 39.0 versus HADS-A≥8: 33.3, p=0.004) to have a significant relationship (
Figure 2).
Discussion
It is well known that depression and anxiety are common comorbidities in the MS population; a survey study from a UK MS registry of 7786 MS patients found that 54.1% and 46.9% scored positive for anxiety and depression symptoms, respectively, on the HADS. This study also found depression and anxiety symptoms to correlate positively, with anxiety symptoms more common in women and in patients with RRMS, whereas depressive symptoms were more common in men.
29 Our study also found a high prevalence of anxiety symptoms on the HADS-A (53.6%) but a lower level of depressive symptoms (34.4%); we also found anxiety and depressive symptoms to correlate strongly with each other.
Early studies examining the relationship between depression and cognitive impairment in MS patients did not find a relationship.
30,31 However, many of these studies had small sample sizes. Furthermore, they often used depression scales that did not account for symptoms that occur due to both MS and depression, that were focused on somatic rather than nonsomatic symptoms, or that used cognitive tests that were not sensitive in the population with MS.
10,11 Contrasting this early research, more recent studies have consistently found a relationship between depression and cognitive impairment. Sundgren et al.
32 separated the Beck Depression Inventory (BDI) into somatic and nonsomatic complaints and found that higher scores on the nonsomatic questions correlated with impaired processing speed, attention, and executive function, whereas somatic symptoms correlated with visual memory, executive function, and processing speed.
32 Arnett et al.
33 found that depressed patients with MS performed worse on the PASAT and SDMT than did nondepressed patients with MS and normal control participants but not on the CVLT. In another study, Arnett et al.
9 found that depressed patients with MS demonstrated impaired working memory compared with nondepressed patients with MS and normal control participants. A meta-analysis of 10 studies in patients with MS with depression found an association between major depression and PASAT scores.
34 There is also evidence that depression and cognitive impairment are not just correlated but that depression worsens cognitive function in patients with MS. Hildebrandt and Eling
35 observed 40 patients with RR MS for 1 year and found that an increase in depressive symptoms during this time frame was associated with impairment on the PASAT and CVLT–Immediate Recall at the 1-year follow-up.
The results of this current study are congruent with previous research yet are incongruent in other ways. Similar to previous studies, we found that the presence of depression was associated with impairment on the SDMT, BVMTR–Delayed Recall, and DKEFS. We did not find a relationship with the PASAT or CVLT2. However, this may be due to differences in our population compared with previous studies. The mean HADS-D was 5.8 with a standard deviation of 3.9; therefore, for those who yielded positive results on the HADS-D, the scores were low, indicative of mild depressive symptoms. Several of the previous studies described above only found a relationship between depression and cognitive impairment in moderate to severe depression.
33,34 Demaree et al.
36 examined this discrepancy specifically by comparing patients with MS with high scores versus patients with low scores on the BDI. This study found that the high BDI group was significantly impaired on the PASAT compared with the low BDI group and the normal control participants. Similar results were found on measures of attention.
36 Thus, although the effect of depression on cognitive impairment in MS has been examined in the past, the exact nature of the relationship is still not fully understood; therefore, our results add to our current understanding.
There is less published literature on the relationship between anxiety and cognitive impairment in MS, despite a high prevalence of anxiety in the population with MS. Certainly the presence of anxiety is coupled with the self-perception of cognitive impairment.
37 Only one study has examined this relationship objectively. Similar to the design of this study, Goretti et al.
14 examined the correlation between self-reported anxiety, using the State-Trait Anxiety Inventory, and cognitive impairment on Rao’s Brief Repeatable Battery of Neurological Tests,
24 examining state anxiety and trait anxiety separately.
14 Similar to our findings, this study found a high rate of anxiety, reporting 64% of the patients with MS as positive for state anxiety, whereas 57% yielded positive results for trait anxiety. Overall, 40% of the patients with MS met criteria for cognitive impairment, defined as failure on at least three tests in the battery, most commonly impaired on measures of processing speed (SDMT, PASAT) and verbal memory (Selective Reminder Test). However, in contrast to our study, this group found that only state anxiety was related to impairment on the SDMT, with a trend toward a relationship with impairment on the PASAT and overall cognitive impairment. There are several reasons why our study results may differ. First, it could be our definition of anxiety. We used the HADS, which has been found to be sensitive and specific for generalized anxiety symptoms in MS patients,
19 whereas Goretti et al.
14 used the State-Trait Anxiety Inventory. Second, we approached our analysis from the opposite direction—we examined those with and without anxiety and compared scores on the cognitive tests, whereas Goretti et al.
14 examined those who were impaired on a cognitive test and compared anxiety scores. Finally, although the population characteristics are similar between our two studies, our population was an English-speaking North American population, but the study by Goretti et al.
14 was based at an Italian center. Therefore, it is possible there are ethnic or cultural differences between our two populations.
There were several limitations to our study. It was a retrospective chart review and thus examined only the association of depression and anxiety symptoms with cognitive impairment and cannot be used to determine a causal relationship. Second, all included participants were referred to the MS Cognitive Clinic for assessment of cognitive function. Thus, either the patient, a family member, or the neurologist suspected cognitive impairment; selection bias may be present. Our study relied on self-reported measures of anxiety and depressive symptoms. Although the HADS is validated for this population, there were inherent limitations to self-reported questionnaires. We did not use more formal diagnostic criteria such as evaluation by a psychiatrist. Furthermore, although depression and anxiety symptoms in this study were associated with different cognitive domains, they also correlated strongly with each other. Therefore, with this retrospective study, it is not possible to fully distinguish between the effects if these two symptoms. Finally, we examined RR MS patients only and thus cannot comment on whether the relationship between anxiety and depression found in this study extends to all types of MS such as secondary-progressive or primary-progressive MS.
Overall, our study demonstrates that both anxiety and depressive symptoms are associated with lower scores, or worse performance, on objective cognitive tests, adding to our current understanding of the effects of mood symptoms on objective cognitive tests in the MS population. This information will help clinicians when evaluating MS patients complaining of cognitive impairment, as it contributes to our understanding of the interplay between depression and anxiety symptoms and cognitive performance. Further research is still needed to determine the causal relationship between the mood disorders and cognitive impairment. However, because there are currently no approved treatments of cognitive impairment in patients with MS—although depression and anxiety can be treated with both pharmacotherapy and other treatment modalities—cognitive complaints may improve if these mood disorders are treated.