A diagnosis of a major mood disorder (major depressive disorder or bipolar affective disorder type I, BP1) is among the strongest correlates of completed suicide, and this risk is elevated early in the illness course (reviewed in
1–3). How this risk is mediated by brain function remains unclear. Altered measures of serotonin function in the prefrontal cortex (PFC) have been found postmortem in suicide completers (reviewed in
4). Patients with major mood disorders have disturbed PFC and subcortical function
5; however, the in vivo correlates of suicidal behavior in these disorders also indicate disturbances in the structure and function of PFC, relative to mood disorder patients without a history of suicidal behavior (reviewed in
6). These include altered structure in the PFC and striatum
7–13 and altered PFC function as measured by functional MRI (fMRI).
14–19How disrupted PFC-based circuit operation contributes to suicide risk in these populations remains uncertain. However, cognitive neuroscience–informed models of PFC function offer a generative framework to address these phenomena. These models describe
control processes, which support cognitive processes such as attention, decision making, thought/language, emotion regulation, and action.
20,21 Proper control and optimal goal attainment depend in part on the detection of conflict in task processes. Conflict occurs when two information processes compete for attention and cognitive resources, such as when there is a need to inhibit a prepotent response tendency in favor of task-relevant response. Resolution of conflict depends on the subject’s capacity to direct attention appropriately and to bias either sensory and/or motor processes, each in favor of the task-relevant process, to optimize goal attainment. Conflict monitoring is mediated by midline frontal regions, prominently including the dorsal anterior cingulate cortex (dACC),
22,23 and may link serotonergic dysfunction to suicidal behavior
24 as an important candidate mechanism underlying suicide risk.
In our published study of patients with recent-onset schizophrenia,
25 we found past suicidal ideation associated with relatively higher conflict-related dACC functional connectivity with medial parietal lobe and striato-thalamic nuclei. In contrast, among those with past suicidal ideation, past suicidal behavior was associated with lower conflict-related dACC connectivity with multiple lateral and medial PFC regions, parietal, and temporal cortical regions. Whether brain-behavior relationships such as these are specific for diagnoses or syndromes or, alternatively, are invariant across these nosologies is an important issue in clinical neuroscience research.
26 We aimed to test our model of control-related PFC function as a correlate of suicidal behavior in psychotic major mood disorders, and to indirectly compare the relationships of past suicidal ideation and behavior to brain function between the present group with mood-related psychotic disorders and the previously reported sample with a primary psychotic disorder. We studied patients with recent-onset psychotic major mood disorders, using a study design similar to that in our study of patients with recent-onset schizophrenia.
25 The mood disorder patients were enrolled from the same first-episode psychosis clinic, evaluated clinically with the same symptom scales, functional MRI protocol, and analyses. Our neuroimaging analyses accounted for the major symptomatic risk factors for suicide in these populations, including psychosis, depression, and impulsivity.
18,25 This afforded tests of the direct relationships of brain function to past suicidal ideation and behavior, which are not mediated by these clinical risk factors. In addition, in our analysis of past suicidal behavior, brain function was analyzed only among subjects who reported past suicidal ideation, allowing us to potentially disambiguate brain function associated with suicidal behavior from that associated with ideation alone, which represents an important issue in suicide-risk research.
27Methods
Subjects
The study was conducted at the Imaging Research Center at the University of California, Davis (UCD) Medical Center. The UCD School of Medicine institutional review board approved all procedures, and written informed consent was obtained from all subjects. Inclusion criteria included age between 18 and 50 years, right-handedness (by Edinburgh Handedness Inventory), and a diagnosis of 296.× with past psychotic episode (by DSM-IV-TR). Exclusion criteria included any neurological illness, uncorrected visual or peripheral motor problems, full-scale IQ below 80 (by the 2-scale Wechsler Abbreviated Scale of Intelligence), comorbid 295.× diagnosis, active substance-related disorder in the 6 months prior to the study, history of self-harm with neurological sequelae, uncontrolled serious medical illness, and established MRI incompatibility. All included subjects had negative tests for illicit drugs in the urine at each study visit.
All patients were recruited as clinically stable outpatients from the UCD Early Diagnosis and Preventive Treatment (of Psychosis) research clinic, with their first psychotic episode occurring within 2 years of study and no hospitalizations or medication regimen changes for at least 2 months prior to study. Past suicidal behaviors each occurred during a major mood episode; however, no subjects were in a major mood episode or acutely psychotic at study. The sample included patients with major depressive disorder (N=9) and BP1 (N=21). The frequencies of medications prescribed at study were as follows: atypical antipsychotics, N=17; antidepressants, N=7; lithium, N=5; and anticonvulsants, N=3. No patients were receiving clozapine or psychotherapies specifically treating suicide risk. Patients were assessed with the Structured Clinical Interview for DSM-IV-TR (SCID). Diagnosticians were SCID-trained master’s- or doctoral-level clinicians, with demonstrated reliability, defined by kappa≥.70, for categorical measures. Symptom measures were determined by a single trained master’s-level rater. We have previously reported in this sample a study of task-related PFC function and its relationship to past suicidal ideation/behavior;
18 the present dACC functional connectivity analysis has not been previously reported.
Clinical Measures
Clinical measure of suicide risk: description and rationale.
The Columbia Suicide Severity Rating Scale (C-SSRS) was used to evaluate past suicidal ideation and behavior. This is a comprehensive, structured interview–based instrument with established validity and internal reliability derived from three multisite studies with diverse patient populations.
28 Three subscales are included. Suicidal Ideation (SI) is an ordinal subscale with items rated on a yes/no basis, including the wish to be dead, the specificity of these thoughts, such as whether they are “active,” with intent and plan. Intensity of Ideation (II) assesses the frequency, duration, controllability, deterrents, and reasons for thoughts of suicide: II item scores are rated on a 1–5 scale and summed for a subscale total score for the present analyses. Suicidal Behavior (SB) is a nominal subscale categorizing past actual, interrupted and aborted attempts to die, and preparatory acts (as yes/no, with event counts); with actual attempts, the potential or actual lethality or medical damage sustained in the attempt is indicated. All C-SSRS items are associated with future suicidal behavior and/or completed suicide.
28Suicidal Behavior is operationally defined here as overt actions with the intent of self-inflicted fatal injury. We used the term “suicidal behavior” as shorthand for this definition and emphasized the initiation of deliberate self-injury with intent to die as the threshold (i.e., a positive response to Actual Attempt, Interrupted Attempt, or Aborted Attempt). We aimed to mitigate the variable influence of the wide range of situational factors that determine whether self-injurious action is completed (with or without death), as these are often unrelated to the subject’s apparent intent or overt behavior. Positive responses restricted to non-suicidal self-injurious behavior and/or preparatory acts or behavior were deemed as not meeting the criterion. The target subjects in the SB model were then dummy-coded as “SB+” or “SB–.”
We also assessed the major symptoms found in these populations that contribute to suicide risk, including psychosis (with the Scale for the Assessment of Positive Symptoms [SAPS]), depression (using a measure validated in psychotic patients, the Calgary Depression Scale [CDS]), and trait impulsivity (with the Barratt Impulsivity Scale, 11th version [BIS-11]). We also measured current manic symptoms with the Young Mania Rating Scale but excluded mania scores from the neuroimaging statistical models, as mania is not an established risk factor for suicide.
29 All symptoms were assessed within two weeks of MRI.
AX Version of the Continuous Performance Task (AX-CPT).
We have previously reported work with the use of this neuroimaging and task paradigm.
18,25 Briefly, fMRI was conducted during performance of the AX version of the Continuous Performance task (AX-CPT), a widely used cognitive control task. Subjects make a “target” response (by two-choice button-press) to the probe letter X only when it immediately follows the cue letter A. All other stimuli require a nontarget response, including trials with probe letter Y and trials in which the probe letter X is preceded by any letter other than A (collectively referred to as B cues). Conflict monitoring occurs particularly on BX trials, in which subjects must use the proper rule to overcome the trained prepotent tendency to make a “target” response to probe letter X. Performance measures for this sample on this task have been reported previously.
18Functional Neuroimaging
Acquisition, preprocessing, and modeling of fMRI data.
fMRI was conducted on a 1.5T GE scanner, and blood-oxygen-level-dependent (BOLD) contrast acquired during single-shot, echo-planar imaging (EPI) with a T2*-weighted sequence. The EPI sequence parameters included TR=2,000 ms, TE=40 ms, flip angle=90°, FOV=220×220 mm, with 24 contiguous slices, 4.0-mm isotropic voxel size, and matrix size=64×64 (extending from 80 mm superior to 16 mm inferior of the anterior commissure–posterior commissure plane). SPM8 was used for preprocessing, with six-parameter linear motion correction (spatial alignment to the first scan of the time-series), coregistration to each subject’s in-plane T2 image, normalization to the standard MNI template (via nonlinear warping, using parameters derived from the subject’s in-plane T2 image), and intensity normalization and smoothing with an 8-mm kernel.
We convolved the canonical hemodynamic response function (HRF) with a series of delta functions placed at each cue and probe onset to model task-related events. To evaluate conflict monitoring, we contrasted neural responses to (high-conflict [BX] probes minus low-conflict [AX] probes). These were analyzed at the subject level as voxel-wise, fixed-effects, then at the group level by random-effects analysis. The resulting group-level conflict-monitoring task contrast map was evaluated at p<.01 for medial frontal activity, and a 7,856-mm3 cluster was identified in the dACC as the seed for the functional connectivity analysis.
Analysis of the relationship of dACC functional connectivity with suicidal ideation and behavior.
We then conducted a bivariate analysis in order to test the hypothesis that altered functional connectivity with the dACC would be associated with past suicidal ideation and behavior in this sample. As with our prior study,
25 we utilized the beta-series correlation method
30 to derive trial-by-trial parameter estimates which were used to correlate time-series between the dACC seed and the rest of the brain. For each subject, coupling between brain regions was measured by computing the bivariate correlations between the time-series of mean β values across all voxels contained in the dACC seed region, and the mean β value from each remaining voxel throughout the brain, independent of other remaining voxels.
To evaluate functional connectivity during conflict monitoring, we contrasted the correlations to (high-conflict [BX] probes minus low-conflict [AX] probes). These were conducted as voxel-wise, fixed-effects analysis at the subject level, then random-effects analysis at the group level. Group-level modeling and statistical contrasts generated for inferential testing yield test statistics at each voxel of the association of past suicidal ideation or behavior with the magnitude of coupling of dACC seed activity with voxel-wise brain activity. The group-level regression model for each task contrast included total scores on scales for depression (CDS), impulsivity (BIS-11), and psychosis (SAPS), and either SI status, II total scores, or SB status, in parallel analyses of the three C-SSRS subscales. The reported contrasts show brain regions where task-related BOLD signal functional connectivity with the dACC seed during conflict monitoring is significantly related to SI, II, or SB, respectively. The SB model was conducted only on SI+ subjects (N=16) to obviate brain effects related to ideation.
27 To determine statistical significance at the group level, we conducted a Monte Carlo simulation, using the AlphaSim program in AFNI. Contrast maps were thresholded at p<.005, and the simulation returned cluster sizes to comprise the empirically determined statistical threshold at p<.05. The cluster thresholds in the contrast maps were as follows for each model: SI, 143 voxels (1,144 mm
3); II, 151 voxels (1,208 mm
3); and SB, 165 voxels (1,320 mm
3).
Results
Subject demographic and clinical characteristics and task performance are reported in
Table 1. No demographic or clinical measures were significantly different between any pairs of subgroups defined by the presence versus absence of past suicidal ideation or behavior.
18 The dACC functional connectivity neuroimaging results are summarized here with brain regions described primarily in functional-anatomic terms; their corresponding anatomic descriptors (gyri/region names and Brodmann areas) are listed in
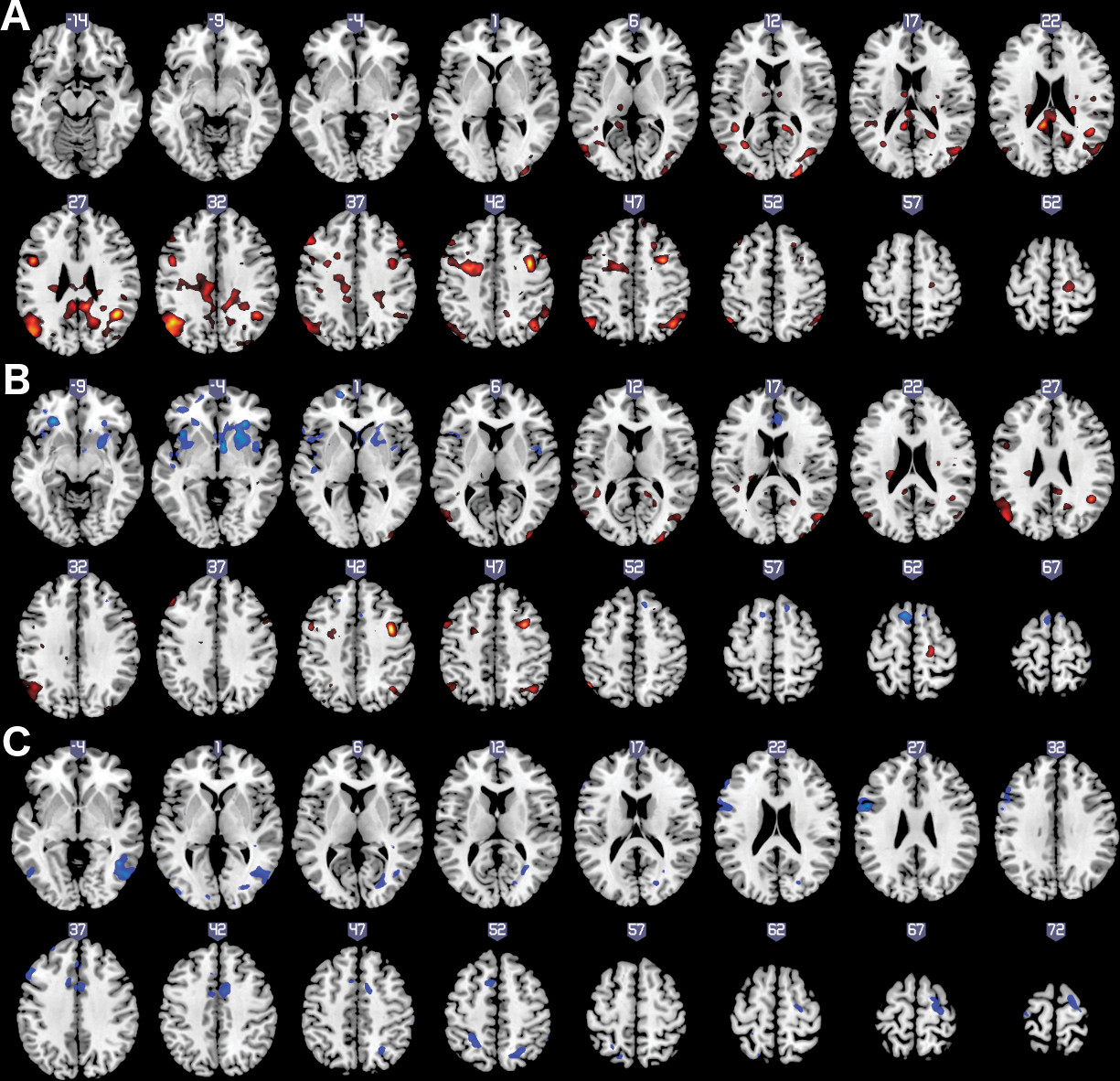
Table 2. The relationship of C-SSRS measures with dACC functional connectivity during conflict-monitoring showed a pattern that differed in relation to suicidal ideation compared to suicidal behavior (
Figure 1 and
Table 2). The experience of past suicidal ideation was related to relatively higher dACC functional connectivity with bilateral dorsolateral PFC and premotor cortex, bilateral dorsal parietal cortex, and inferior temporal-occipital cortex. The Intensity of Ideation subscale was also related to higher dACC functional connectivity, but primarily with posterior cortical regions in the temporal, parietal, and occipital cortex, and relatively more restricted regions in the bilateral premotor cortex. In addition, however, the Intensity of Ideation scores were also associated with relatively lower conflict-related dACC connectivity with ventral forebrain regions, including in the bilateral ventrolateral PFC (VLPFC), and adjacent regions including the orbitofrontal cortex (OFC), rostral insula, and striatal lenticular nuclei (putamen and globus pallidus), plus circumscribed, dorsal cortical regions in the left supplementary motor area (SMA) and right somatosensory cortex. In contrast, past suicidal behavior showed exclusively lower dACC functional connectivity, with left dorsolateral PFC (DLPFC) and frontal motor areas including bilateral premotor cortex, right SMA, and pre-SMA adjacent to the dACC seed, and posterior cortical regions in inferior temporal cortex and superior parietal cortex.
Discussion
In this study of patients with recent onset of major mood disorders with psychotic features, we found that a history of suicidal ideation was related to relatively higher dACC functional connectivity with dorsal fronto-parietal circuits, as well as lower conflict-related dACC connectivity with bilateral VLPFC and adjacent fronto-striatal regions. Among those with past suicidal ideation, past suicidal behavior was associated with lower dACC functional connectivity with DLPFC and premotor cortex, as well as temporal-parietal cortex. It is important to note that these associations were independent of the major symptoms that represent established risk factors for suicide in these disorders, including depression, impulsivity, and psychosis, which themselves are associated with disturbed brain function. It therefore appears that the present findings identify altered frontal-based brain function that directly relates to past suicidal ideation and/or behavior, as a key measure of long-term suicide risk in these patients.
These findings are generally consistent with prior reports of brain function related to past suicidal behavior in patients with mood disorders. Remitted major depressive disorder patients exhibit relatively higher task-related activity in ventral,
15,31 lateral, and medial PFC sectors
19,31 in association with suicidal behavior, somewhat analogous to our findings of greater conflict-related dACC connectivity with lateral PFC sectors, associated with Suicidal Ideation and Intensity of Ideation subscales of the C-SSRS.
However, the literature is mixed, with some reports of decreases in task-related activity in frontal and subcortical regions in patients with past suicidal behavior.
16,17,31 In a somewhat analogous manner, we found that a past history of suicidal ideation (vs. absence of this history) was associated with relatively stronger conflict-related dACC connectivity with dorsal and lateral PFC sectors that support cognitive control; in contrast, the intensity of that suicidal ideation was associated with relatively lower dACC connectivity with the ventral forebrain circuitry that includes VLPFC, OFC, insula, and striatum. This latter (ventral) circuitry occupies a “middle ground” in the cortical-subcortical processing pathway that translates goals into actions, including inhibitory action control (right VLPFC),
32,33 reversal learning (lateral OFC),
34 and subjective responses to the environment and interoceptive cues (rostral insula).
35 Among mood disorder patients, impaired dACC-mediated engagement of these intermediate PFC subnetworks in the face of heightened DLPFC-mediated sensitivity to task conflict may lead to inadequate inhibitory regulation over subcortical regions, resulting in the emergence and dysregulation of suicidal ideation, compared with those who share a diagnosis or symptoms but lack suicidal ideation/behavior or, alternatively, those who experience suicidal ideation but in a less-intense and better-controlled manner. We also found that a history of overt suicidal behavior was associated with lower dACC connectivity with lateral PFC regions, including DLPFC and premotor cortex. This suggests that a critical difference between those mood disorder patients who merely experience suicidal ideation, versus those who initiate overt suicidal behavior, may relate to a threshold of disturbed communication between dACC as a conflict-monitoring center and the lateral PFC sectors such as DLPFC and frontal motor areas that use this dACC input to adjust and optimize goal-related behavior. A divergence between brain functioning and suicidal ideation versus overt suicidal behavior was also found in a recent study
36 in which medial thalamus activation in a go/no-go task was correlated with current suicidal ideation scores, yet no significant differences in brain function were observed between depressed patients with versus without past suicide attempts. These findings underscore the importance of disambiguating suicidal ideation and behavior when testing relationships with brain function. The issue of brain laterality effects remains understudied as well. In one study,
31 several lateralized effects related to decision making were seen among the depressed group with past suicidal behavior, including increases in the left OFC and VLPFC to facial expressions of anger, as well as in the right OFC, DPFC, and ACC in response to winning trials, and decreases in the left DLPFC to risky choices and in the right ACC to sad faces.
Interestingly, the brain topographies observed in the present mood disorder sample differed from those we found in patients with recent-onset schizophrenia whom we studied with identical clinical, experimental, and analytic methodologies.
25 In the schizophrenia group, suicidal ideation was associated with higher conflict-related dACC functional connectivity in medial parietal cortex and striato-thalamic regions, and suicidal behavior with lower dACC connectivity with ventrolateral and dorsomedial PFC regions, plus medial parietal cortex. These differences suggest that how the brain responds to conflict is altered in a divergent manner in association with suicidal ideation or behavior for these two clinical populations, despite similar symptom profiles among the two groups. This finding may have implications for transdiagnostic perspectives emerging from the National Institute of Mental Health Research Domain Criteria initiative
26; the question of whether brain-behavior relationships are linked to psychiatric diagnoses or syndromes warrants further study.
Study Limitations
A few study limitations are apparent. First, the size of the subsample with past suicidal behavior was relatively modest. Second, the sample included recent-onset unipolar and bipolar patients. In this study, our rationale in combining patients with these two diagnoses was to distinguish a sample with mood-related psychotic disorders from the prior sample with primary psychotic disorders. In addition, up to 35% of those initially diagnosed with psychotic depression go on to develop a bipolar disorder,
37–39 suggesting that some of the present patients with unipolar psychotic depression may emerge with a bipolar condition. Nonetheless, the study enrollment limits the strict
DSM-diagnostic specificity of the findings. Third, the subjects were actively taking psychiatric medications, which may affect brain function, suicide risk, or the relationship between the two. Fourth, the fMRI scanning procedure did not cover ventral limbic brain regions such as the amygdala and hippocampus. Therefore, the role of fronto-limbic function in suicide risk in major mood disorders remains to be studied within a model of PFC-based cognitive control.