Time perception is a complex, yet essential, ability of the human brain and cognitive processing. This ability is also important in everyday life behavior, for instance, when crossing a busy street, playing an instrument, or playing sports. Time perception has been found to be compromised in patients with Korsakoff’s syndrome. The syndrome refers to a neurocognitive disorder that is related to chronic alcohol abuse coupled with a thiamine deficiency.
1,2Clinical observation has long suggested that anterograde amnesia, a hallmark of the syndrome, produces a severe distortion of the sense of time. Korsakoff
3 himself already noted that memory for temporal information is often more severely affected than memory for events. Similarly, Sacks
4 described how an individual with Korsakoff’s syndrome was unaware of the passing of decades. Clinical observations of compromised time perception in Korsakoff’s syndrome have also been supported by empirical research. In a relevant study, Kinsbourne and Hicks
5 instructed participants with Korsakoff’s syndrome to read single digits and to verbally estimate their reading time. Results demonstrated underestimation of intervals beyond 30 seconds in these participants. Similar findings were reported by Shaw and Aggleton,
6 who evaluated time reproduction and time estimation of intervals between three and 96 seconds in participants with Korsakoff’s syndrome. On the time reproduction evaluation, the authors demonstrated an interval of time by saying “start” and “stop,” and the participants had to reproduce that interval by also saying “start” and “stop.” In the time estimation task, the participants were given the time in seconds and asked to produce this interval. Results showed a bias to underestimate longer intervals in these participants. These findings were observed in another study in which conditions of time estimation and time reproduction of intervals between 10 and 120 seconds were assessed.
7 In this study, participants with Korsakoff’s syndrome were asked to read numbers aloud and to estimate how many seconds each trial had taken, whereas on the time reproduction evaluation the participants were required to indicate when they thought a predetermined time interval was over. Findings showed underestimation of durations as intervals grew longer. Deficits in time perception were also observed in Korsakoff patients by Brand and colleagues,
8,9 who asked Korsakoff patients to estimate the duration of specific events (e.g., “How long is the duration of a morning shower?”). Participants showed a tendency to underestimate the duration of these events.
In the aforementioned studies, participants were instructed in advance that they had to estimate or reproduce time intervals. This prospective timing approach can be contrasted with retrospective timing tasks in which participants are not aware of the timing until the end of the task.
10 Evaluation of both prospective and retrospective timing has been recently done in patients with Alzheimer’s dementia.
11 Here, participants performed four retrospective timing tasks: deciding whether words were abstract or concrete for 30 seconds, filling connected squares for 60 second, deciding whether words were animal or object names for 90 seconds, and reading a text about mushroom picking for 120 seconds. These intervals were chosen because estimating delay less than 30 seconds seems to be preserved in Alzheimer’s patients.
12 Prior to each task, the experimenter explained the lure purpose of each task (e.g., filling connected squares) and did not mention that timing was in any way relevant until the tasks were finished. In the prospective tasks, the participants had to read aloud a series of numbers for four time intervals (i.e., 30, 60, 90, and 120 seconds) and were explicitly instructed before the task that they had to estimate the duration of reading. For both the prospective and retrospective tasks, participants with Alzheimer’s disease showed underestimation of the time intervals (for similar findings, see El Haj et al.
13).
Because research on time perception in Korsakoff’s syndrome was concerned with prospective timing, the present study aimed to assess retrospective timing in the syndrome. The second aim of our study was to explore the cognitive underpinnings of timing deviations in the syndrome. Timing deviations are intimately linked with memory distortion, an assumption that can be supported by a study showing a relationship between underestimation of time intervals and difficulties in subjectively reliving past events for those with Alzheimer’s disease.
11 According to Friedman and Janssen,
14 the difficulty of retrieving events from a certain interval of time leads to the impression that this interval is empty. Hence, by leaving fewer events to be remembered, episodic memory decline induces the feeling of “empty” time intervals and, thus, the underestimation of elapsed time. It is worth noting, however, that temporal events can be considered as represented along a mental time line and that the sensorimotor system is linked to that representation.
15–17In addition to memory, executive functions may also mediate timing deviations in Korsakoff’s syndrome. This assumption is supported by a neuropsychological case study showing important overestimation of time intervals in a patient with prefrontal dysfunction.
18 Similar clinical reports have suggested a key role for the prefrontal cortex in processing temporal information. Studies have described overestimation for long intervals (60 seconds) in patients with left or right prefrontal lesions.
19,20 These reports are in line with neuroimaging findings, showing activation of the (right) prefrontal cortex, during time processing (for a review, see Grondin
10). Prefrontal dysfunction has also been demonstrated in Korsakoff’s syndrome (for a review, see Oscar-Berman
21), and negative correlations have been observed between time deviations and performances on cognitive tests assumed to assess frontal-executive functions.
6The relationship between time estimation and both memory and executive function can be illustrated with the attentional gate model,
22 according to which time perception requires an attentional gate controlling pulses that are emitted by a pacemaker. When attentional processes are solicited for timing, the gate allows more pulses to head to an accumulator that counts the number of pulses, a number referring to the duration of an interval. When a target interval must be reproduced, the numbers of ongoing pulses that are counted heading into the accumulator are compared with previous pulse counts, which are stored in working memory and long-term memory.
In summary, research on time perception in Korsakoff’s syndrome has been concerned with prospective timing. Here, we aim to extend this research by investigating retrospective timing. Another aim of our study was to explore the cognitive underpinnings of retrospective timing deviations in the syndrome. In line with the literature on prospective timing deviations,
5–9 we expected deviations in retrospective timing to be present in these patients. We also expected a significant relationship between deviations in retrospective timing and deficits in both memory and executive functions.
Methods
Participants
Eighteen participants with anterograde amnesia, diagnosed with Korsakoff’s syndrome, were recruited from inpatient and daycare facilities in Lille, France (11 women and seven men; mean age=56.78 years [SD=5.65]; mean years of formal education=9.50 [SD=3.18]). These participants underwent a full examination by experienced psychiatrists as well as neuropsychologists, confirming the
DSM-IV23 criteria for alcohol-induced persisting amnestic disorder. Amnestic syndrome was observed in the initial diagnosis, a finding that was confirmed by our neuropsychological examination (see below). All participants had an extensive history of alcoholism and nutritional depletion, notably thiamine deficiency, and were in a chronic (more than one year postonset) and stable condition, but none were in the confusional Wernicke psychosis at the time of testing or had signs of alcohol-related dementia.
24 Exclusion criteria were other neurological (e.g., head injury or epilepsy) or psychiatric (e.g., psychosis, major depression) disorders interfering with the testing procedure.
25 Patients were not under psychiatric treatment or using psychotropic drugs at the time of the study.
As a comparison group, we recruited 20 volunteers from the local community without previous or current substance addiction and without neurological or psychiatric history (10 women and 10 men; mean age=55.40 years [SD=5.19]; mean years of formal education=10.00 [SD=4.10]). The neuropsychological performance of controls was verified with an evaluation of memory and executive functions (described below). These participants were matched with the Korsakoff participants according to sex distribution [χ2(1, N=38)=0.47, p>0.10], age [t36=0.78, p>0.10], and educational level [t36=0.41, p>0.10].
Participants were not remunerated. All participants provided written informed consent to participate and were able to withdraw whenever they wished. The study was conducted according to the provisions of the Declaration of Helsinki.
Procedure
Neuropsychological assessment.
Neuropsychological characteristics of all participants were evaluated with a battery tapping general cognitive functioning, episodic memory, spans, flexibility, and inhibition. Scores are summarized in
Table 1.
General cognitive functioning.
We used the Mini-Mental State Examination (MMSE)
26 to assess overall cognitive function (scoring range between 0 and 30).
Episodic memory.
We used the French version of the Selective Reminding Task of Grober and Buschke.
27 For this task, participants had to memorize 16 words, each of which describes an item that belongs to a different semantic category. Immediate cued recall was followed by a distraction phase, during which participants had to count backward from 374 in 20 seconds. This distraction phase was followed by two minutes of free recall, and the score from this phase provides a measure of episodic recall (16 points maximum).
Executive function.
Working memory updating: Central executive of working memory was assessed with spans.
28,29 Participants had to repeat a string of single digits in the same order (i.e., forward spans) or in the inverse order (i.e., backward spans).
Flexibility: The Plus-Minus task includes three lists, each containing 20 numbers. On List 1, participants were instructed to add one to each number. On List 2 they were instructed to subtract one from each number. On List 3, they were instructed to add and subtract one alternately. The flexibility score (in seconds) referred to the difference between the time participants needed to complete List 3 and the average time that participants needed to complete Lists 1 and 2.
Inhibition: The French Stroop Color-Word Test
30 consists of three subtests: word reading, color naming, and color-word interference. In the word-reading subtest, participants had to read 100 words printed in black ink, all words naming colors. In the color-naming subtest, they had to name the color of 100 colored-ink squares. In the color-word interference subtest, participants had to name the color of 100 color-words printed in incongruously colored ink (i.e., the word “red” was written in blue ink). The inhibition score (in seconds) is the completion time for the interference condition minus the average completion time for word reading and color naming.
Time perception.
We used verbal estimation to replicate procedures of previous research on time perception in Korsakoff’s syndrome.
5,6 Four time intervals were assessed (i.e., 15, 30, 45, and 60 seconds); these intervals were also chosen to replicate procedures of research on time perception in Korsakoff’s syndrome.
5,6,8,9 Each retrospective interval consisted of a different activity, randomized across participants: reading three-digit numbers aloud (15 seconds), filling connected squares (30 seconds), deciding whether words were abstract or concrete (45 seconds), or reading aloud a text about mushroom picking (60 seconds). All tasks were paper-and pencil-based. Prior to each task, the experimenter explained the stated purpose of the procedure (e.g., categorizing words as representing abstract or concrete things). He then asked the participants whether they were ready to perform the task. When the answer was affirmative, he gave the signal “Go”; at the end of the corresponding time interval, he gave a “Stop” signal and immediately asked the participants, “How many seconds did the task last?” At the start of the “Go” signal, the experimenter activated a laptop stopwatch. At “Stop” the stopwatch was deactivated. The screen of the laptop was hidden from the participant’s view so that she or he was not aware of timing. For the same purpose, the experimenter was careful to give the “Go” signal and to keep working on the laptop during all the study tasks. All the participants had the following (stated purpose) instruction: they had to complete the assignments as accurately as possible. Another precaution that was taken to minimize guessing for the real purpose of these tasks was intermixing them with neuropsychological tests, during which participants were not asked to provide time estimation. To the same end, we assessed time perception and neuropsychological battery during two sessions. Each session lasted between 30 and 45 minutes. Sessions were spaced one week apart on average (two timing tasks during each session).
Performance on time perception tasks was scored by 1) the time estimation as reported by the participants and 2) the absolute error value, that is, the difference between predicted and actual time, regardless of the sign. The absolute error is considered one of the most sensitive measures of time perception, because it assesses the extent to which participants’ responses vary from the event’s actual duration, regardless of whether the response is an over- or an underreproduction.
31Analyses
We carried out a mixed analysis of variance with group (Korsakoff participants and controls) as the between-participants factor and condition (15, 30, 45, and 60 seconds) as the within-subject factor. Prior to the analysis, we checked for normal distribution of data with Shapiro-Wilk tests (used because of the small sample sizes). Partial eta-squared values are reported as effect sizes, where η
p2 <0.01 indicates a small size effect, η
p2 >0.14 indicates a medium size effect, and η
p2 > 0.24 indicates a large size effect.
32 We also calculated Pearson product-moment correlation coefficients to analyze the relationships between absolute errors, memory, and the executive measures. Correlational analyses were carried out on compound scores based on standardized z scores. Episodic raw scores were transformed into z scores, an executive score was calculated as the mean of the four executive z scores (i.e., forward span, backward span, flexibility, and inhibition), and an absolute errors score was calculated as the mean of the four z-error scores (i.e., absolute errors on the 15, 30, 45, and 60-second intervals).
Results
Shorter Time Estimation in Korsakoff Participants Than in Controls
Table 2 shows the time estimation and absolute error values in the four timing tasks. With respect to time estimation, a significant group effect was found, F(1, 36)=11.99, p<0.01, η
p2=0.25; time estimation, as reported by participants with Korsakoff’s syndrome (mean=24.62 [SD=11.33]), was shorter than the time reported by controls (mean=32.20 [SD=7.12]). The main effect of condition was also significant, F(3, 108)=47.15, p<0.001, η
p2=0.57. Time estimation was shorter for the 15-second interval (mean=11.95 [SD=5.51]) than for the 30-second interval (mean=20.62 [SD=8.91]), t
37=4.89, p<0.001; time estimation was also shorter for the 30-second interval than for the 45-second interval (mean=36.06 [SD=14.81]), t
37=6.05, p<0.001, and the 45-second estimate was lower than the 60-second estimate (mean=45.03 [SD=20.01]), t
37=2.08, p<0.05. The interaction effect between group and condition was not significant (F<1).
Because it is of interest to investigate whether time estimation was significantly shorter than real time, we compared retrospective timing with the actual duration and compared prospective timing with the actual duration using paired-sample t tests. Analyses showed that the estimation of the duration of the retrospective tasks in Korsakoff participants (mean=24.75 [SD=5.58]) was significantly shorter than the actual durations of these tasks (mean=37.50 [SD=00.00]), t17=8.69, p<0.001. Time underestimation (mean=32.20 [SD=7.42]) was also observed in controls, t19=3.19, p<0.01.
When we considered absolute errors, a significant group effect was observed, F(1, 36)=10.38, p<0.01, η2=0.22; larger absolute errors were present for participants with Korsakoff’s syndrome (mean=15.11 [SD=9.69]) than for controls (mean=11.22 [SD=7.01]). The main effect of condition was also significant, F(3, 108)=22.99, p<0.001, ηp2=0.39. Smaller absolute errors were observed for the 15-second interval (mean=5.19 [SD=3.87]) than for the 30-second interval (mean=11.38 [SD=6.98]), t37=4.76, p<0.001, for the 30-second interval in comparison with the 45-second interval (mean=15.42 [SD=6.73]), t37=2.38, p<0.05, and for the 45-second interval in comparison with the 60-second interval (mean=21.18 [SD=12.35]), t37=2.19, p<0.05. The interaction effect between group and condition was not significant (F<1).
Significant Relationship Between Memory Decline and Timing Deviations
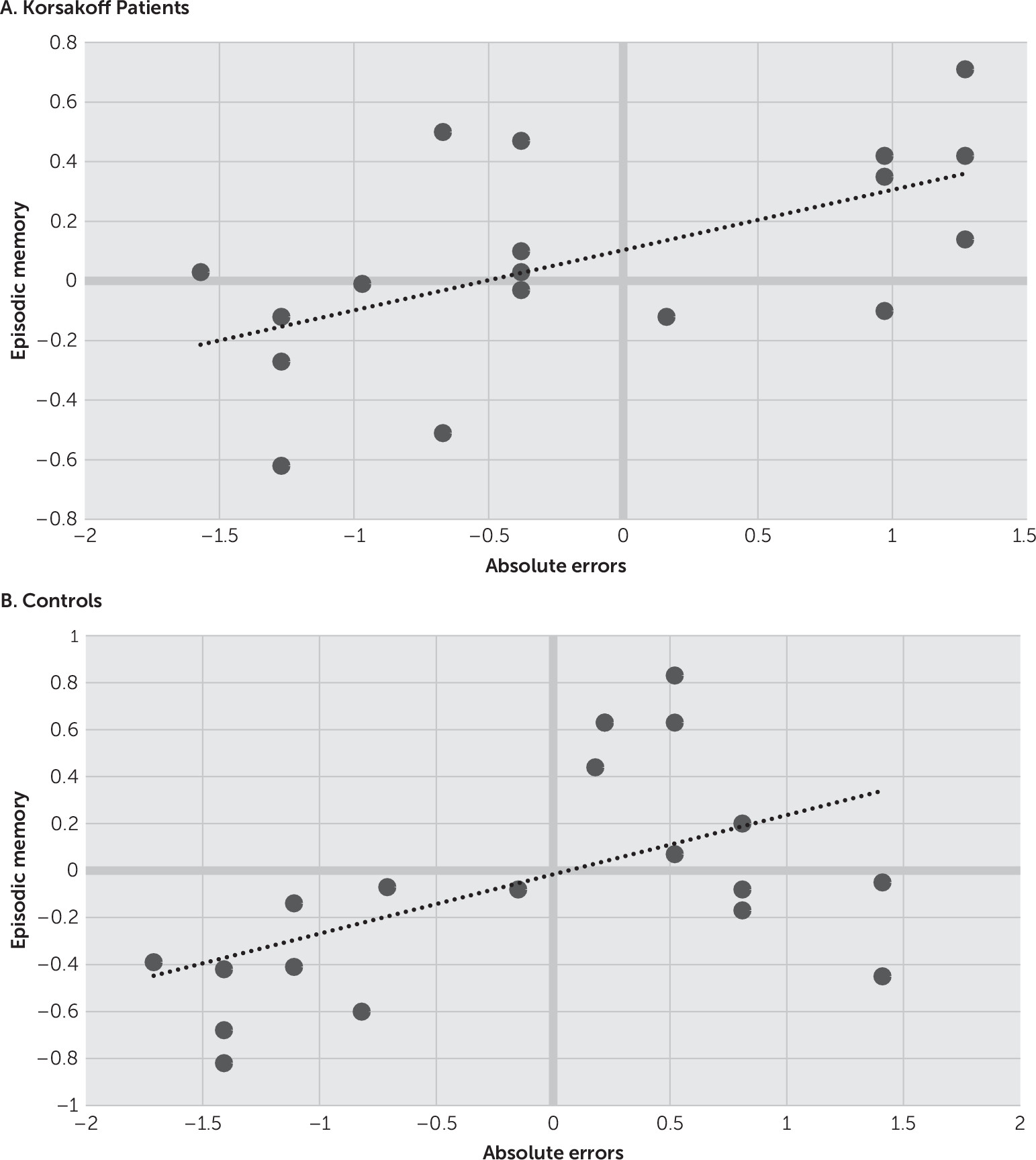
Correlations are depicted in
Figures 1 and
2. Analysis revealed significant correlations between episodic memory function and absolute errors for both the Korsakoff participants (r=0.57, p<0.05) and the controls (r=0.53, p<0.05). Significant correlations were also observed between absolute errors and executive functions for both the Korsakoff participants (r=0.53, p<0.05) and the controls (r=0.51, p<0.05).
A stepwise regression analysis was performed in which absolute errors were the dependent variables and working memory spans, shifting performance, and inhibition performance were entered as predictors. This analysis showed that inhibition was the main and only variable predicting the size of the absolute errors for both the Korsakoff participants (adjusted R2=0.53, F=6.43, p<0.05) and the controls (adjusted R2=0.51, F=6.21, p<0.05).
For convenience, we also report the correlations between performances on the MMSE, the Grober and Buschke task, the forward and backward span, the Plus-Minus task, and the Stroop test for the Korsakoff patients. Analyses showed no significant correlations between the MMSE and any of the other cognitive tasks. Significant correlations were observed between the forward span and the backward span (r=0.45, p<0.05), the backward span and the Stroop task (r=–0.51, p<0.01), the backward span and the Plus-Minus task (r=−0.44, p<0.01), the backward span and the Grober and Buschke task (r=0.47, p<0.05), the forward span and the Grober and Buschke task (r=0.44, p<0.01), and the Stroop and the Plus-Minus tasks (r=0.54, p<0.01); all remaining correlations were not significant.
Discussion
This article investigates retrospective time perception in Korsakoff’s syndrome. When asked to estimate the duration of different performed activities, the Korsakoff participants showed underestimation of the elapsed time. Larger absolute errors reflecting the degree of deviation from the actual time were also observed for Korsakoff participants in comparison with those for controls. Absolute errors were correlated with episodic memory function and executive function.
Research using prospective timing tasks has demonstrated that Korsakoff patients tend to underestimate time intervals
5–7; our findings replicate these findings using retrospective timing. Time deviations, as observed in our participants, were also correlated with their episodic memory performance. Episodic memory allows retrieving events from a particular interval, and difficulty of retrieving these events may lead to the impression that that interval is shorter than the actual time (i.e., underestimation of time).
14 Episodic memory also allows reconstruction of the order of events, which contributes to the estimation of time. Episodic memory decline, as present in Korsakoff’s syndrome, may result in difficulties to retrieve the temporal order of events and thus to time distortions. Our findings can be compared with those of Mimura et al.,
7 who found that Korsakoff patients were impaired on verbal estimation and time production for intervals beyond 30 seconds, despite having an intact subjective tempo for time passing. However, Mimura et al.
7 did not find any significant correlations between time distortions and delayed memory measures in Korsakoff patients, probably because of a floor effect on these memory measures.
Besides correlation with episodic memory, time deviations for our Korsakoff participants showed correlation with executive functions. This correlation converges with that in a study showing a relationship between time deviations and compromise on cognitive tests thought to assess frontal functions.
6 Neuropsychological research shows timing deviations in patients with prefrontal lesions,
18–20 and neuroimaging studies have demonstrated activation of the prefrontal cortex, particularly the right prefrontal cortex, during processing of time (for a review, see Grondin
10). Because Korsakoff’s syndrome can be associated with gray-matter abnormalities in the prefrontal cortex, most notably in the dorsolateral prefrontal region (for a review, see Oscar-Berman
21), these frontal abnormalities can be suggested as support for the correlation between time deviations and executive function in our Korsakoff participants. Our regression analysis showed that, of the executive measures, only inhibition predicted time deviation in both the Korsakoff participants and the controls. These findings are in agreement with research suggesting that individuals with poor performance on inhibition tasks show less efficient timing performance than do those with more intact inhibition ability.
33,34 In a similar vein, Brown et al.
35 explored bidirectional interference between performance on the Stroop task and serial five-second production in cognitively unimpaired participants. Bidirectional interference was observed: relative to single-task conditions on the Stroop task, interval productions became longer and more variable under the interference condition. Concurrent timing also lengthened the response times on the Stroop task.
The relationship between time estimation and both memory and executive functions can also be illustrated with the attentional gate model.
22 According to this model, memory is constantly being updated when reproducing target intervals, which may be the case for the time estimation of elapsed intervals in our study.
Regarding our procedures, one may argue that, after providing a retrospective judgment, our participants were likely to suspect that further time judgments would be required and the paradigm thus became prospective. Although this fact is a major challenge for retrospective timing, which may explain the paucity of research in this domain regardless of the population, our procedures aimed to take this confound into account by spacing tasks one week apart and (two timing tasks per week) by varying the nature of the retrospective tasks. For the same aim, the timing tasks were intermixed with tasks from the neuropsychological assessment, and the participants were instructed to complete the (neuropsychological and timing) assignments as accurately as possible.
As for the correlations between the cognitive tasks for the Korsakoff participants, our analyses showed significant correlations between working memory spans and most of the other cognitive tasks, which may be explained by a shared underlying executive function in these participants. However, no significant correlations were overserved between the MMSE and any of the other tasks, probably because of the relatively high performance on the MMSE, which is relatively insensitive to the deficits seen in Korsakoff patients.
36One suggestion for future research would be to replicate our retrospective procedures on intervals longer than 60 seconds, this in light of research suggesting large time deviations for long intervals.
6 Another suggestion is to assess episodic memory with a test other than the memory task of Grober and Buschke. The latter task assesses memory for items rather than memory for events that occurred in a particular time and space. This issue is important, because Tulving
37 has defined episodic memory as the system that allows remembering personally experienced events and traveling backward in time to re-experience those events.
Time perception is a fundamental cognitive process, considering the fact that all brain and human activities include a temporal organization, at a simple or complex level. Still, despite its relevance to human behavior and cognitive function, time perception still is a poorly studied field and possibly a neglected phenomenon in Korsakoff’s syndrome; there is a need for more studies to understand the nature of timing deviations and the influence of these deviations on everyday life activities in the syndrome.
Acknowledgments
The authors thank Gaëlle Cool, Florence Guidez, Martine Oswald, Stéphanie Potier, and Emilie Wawrziczny for their assistance with patients’ recruitment and data collection.