A Systematic Review of Neuropsychiatric Symptoms and Functional Capacity in Huntington’s Disease
Abstract
Objective:
Methods:
Results:
Conclusions:
Methods
Theoretical Framework
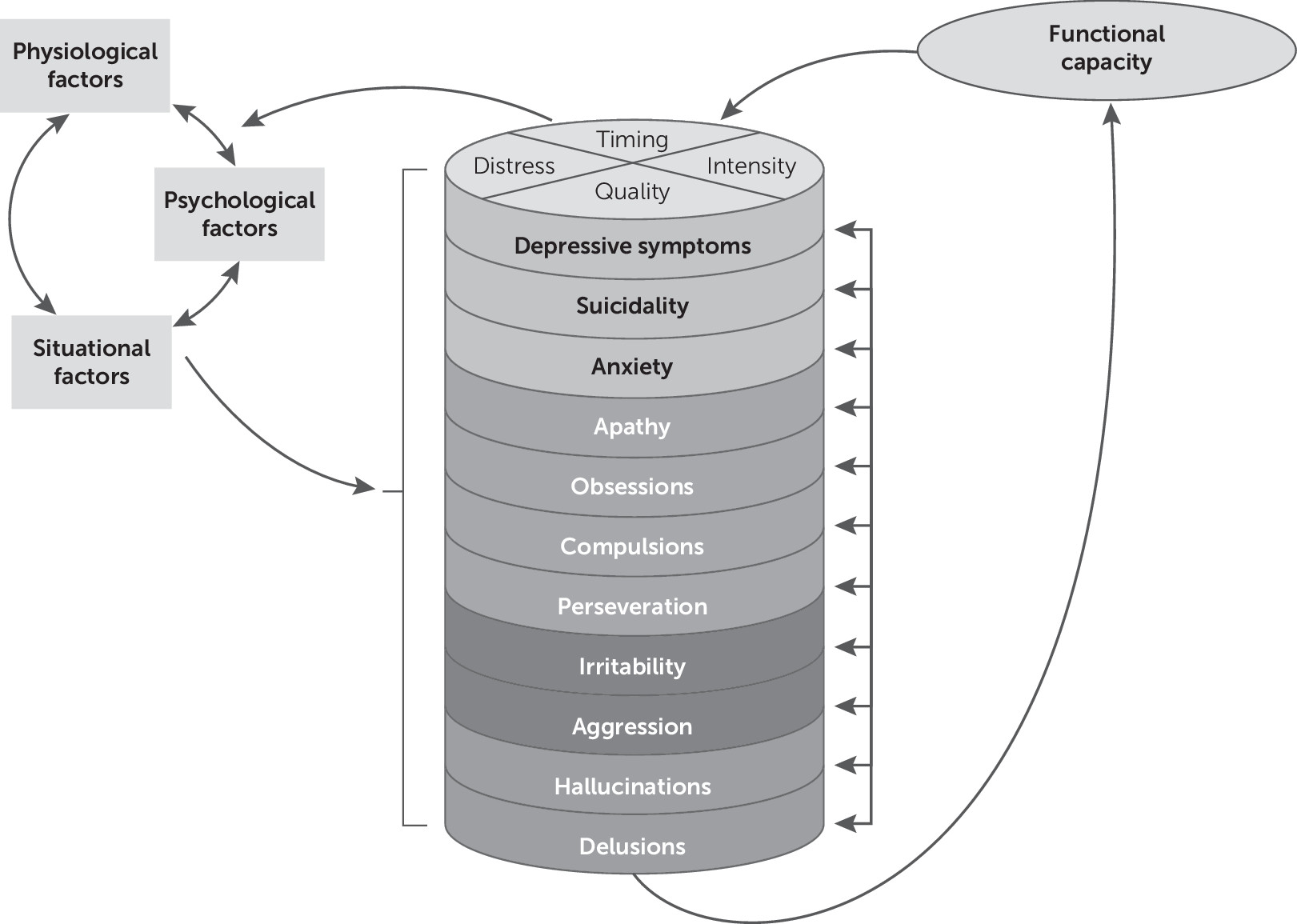
Systematic Review
| Study | Design | Neuropsychiatric symptoms measured for analysis with function | Instrument for neuropsychiatric measurement | Term used for function | Instrument to measure functional capacity | Statistical method for association between psychiatric and functional variables | Results and conclusions related to neuropsychiatric symptoms |
|---|---|---|---|---|---|---|---|
| Anderson et al. (25) | Cross-sectional, retrospective (HSG data set, patients with Huntington’s disease, N=1,642) | Obsessive and compulsive symptoms | UHDRS-b (single item) | Functional capacity | UHDRS TFC | t test | Patients with obsessive-compulsive symptoms (N=446) had significantly worse functional scores than patients without obsessive-compulsive symptoms (N=1,196) (t=3.47, p<0.001). Patients with high obsessive-compulsive symptoms (highest 25% of the O-C obsessive-compulsive symptoms group, N=121) had significantly worse functional scores than patients with low obsessive-compulsive symptoms (lowest 25% of the obsessive-compulsive symptoms group, N=116) (t=4.99, p<0.001). |
| Banaszkiewicz et al. (2) | Cross-sectional, prospective (patient-caregiver dyads, N=80) | Aggression, apathy, depression, irritability, anxiety, and psychosis | UHDRS-b (measured with single items except for psychosis, which included hallucinations and delusions); HAM-D | Functional disability | UHDRS FAS | Simple linear and multiple regression | On simple regression, depression and apathy were significantly negatively associated with functional score. Irritability, aggression, anxiety, and psychosis subscores were not significantly associated with functional score. On multiple regression, apathy was an independent predictor of functional disability. |
| Beglinger et al. (30) | Cross-sectional, retrospective (HSG data set, patients with Huntington’s disease, N=265) | Depression | UHDRS-b (single-item measure of depression) | Functional capacity | UHDRS TFC and FAS | logistic regression (only with FAS) | Depression was significantly related to loss in ability to engage in usual employment on the FAS (p<0.01). |
| Eddy and Rickards (26) | Cross-sectional, prospective (patients with Huntington’s disease, N=25, healthy control subjects, N=20) | Depression, anxiety, and apathy | PBA subscales (single items) | Functional capacity | UHDRS TFC | Spearman correlations | Depression, anxiety, and apathy scores were not significantly associated with functional capacity scores. |
| Epping et al. (23) | Cross-sectional, retrospective (patients with prodromal Huntington’s disease, N=803, gene negative patients, N=223) | Depression | BDI-II | Functional capacity | Shoulson and Fahn TFC | Analysis of covariance | TFC score decreased with increased depressive symptoms (although the TFC decrease from minimal to severe depression groups was only 1 point). |
| Fritz et al. (3) | Cross-sectional, retrospective (baseline data from larger longitudinal study used; patients with Huntington’s disease: prodromal, N=193, early-stage, N=187, and late-stage, N=91) | Apathy | PBA-s (single item) | Functional status | UHDRS TFC, FAS and IS (composite score of clinician-rated functioning created: scores were recoded to ensure that higher scores indicated better outcomes; individual scores standardized to a mean of 0 and standard deviation of 1; standardized scores were summed for each composite score, then sum scores were scaled with a mean of 0 and standard deviation of 1 so that all composite scores were on the same scale) | Multiple linear regression | There was no statistically significant association between apathy and clinician-rated functioning. |
| Hamilton et al. (32) | Cross-sectional (patients with Huntington’s disease and their informants, N=22) | Apathy, executive dysfunction, and disinhibition | Frontal Lobe Personality Scale | functional decline/ functional disability | HD-ADL, TFC (citations for UHDRS and Shoulson and Fahn TFC) | Simple linear regression | Composite apathy/executive dysfunction score (these subscales were combined because they were highly correlated, r=0.83, p<0.001) was significantly related to decline in activities of daily living, even after controlling for motor and cognitive deficits. |
| Hubers et al. (31) | Cross-sectional, prospective (N=152 patients with Huntington’s disease, gene-negative control subjects, N=56); longitudinal data were gathered but not used to examine relationships of interest | Suicidality | PBA (single item) | Global functioning | UHDRS TFC (Shoulson and Fahn TFC also cited) | Binary logistic regression | The difference in numbers of Huntington’s disease patients who were nonsuicidal and suicidal with TFC scores <10.5 was not statistically significant. |
| Marder et al. (27) | Longitudinal, retrospective (patients with Huntington’s disease, N=960) | Mood, anxiety, aggression, psychosis, and other behavioral abnormalities | UHDRS-b | Functional decline | UHDRS TFC and IS | Mixed-effects model to determine influence of disease characteristics and covariates on change in slope of TFC and IS | Increased depression factor (depression/anxiety) at baseline was associated with faster decline on Independence Scale scores (p=0.03). No other baseline psychiatry-related factors had a significant influence on the slope of the IS or TFC. |
| Mayeux et al. (24) | Cross-sectional, retrospective chart review (patients with Huntington’s disease, N=48); Longitudinal analyses were included but not for relationship of interest to this review | Depression | BDI; BPRS | Functional capacity | Shoulson and Fahn TFC | Simple linear regression, partial correlations | Depression scores from BDI and BPRS were significantly negatively correlated with the functional capacity score on simple regression. On partial correlation, the BDI/BPRS scores (seemingly combined) contributed independently to the determination of functional capacity, but it was no longer associated with functional capacity on reanalysis of the BPRS after removing somatic components. |
| Nehl and Paulsen (1) | Cross-sectional, retrospective (patients with Huntington’s disease, included in regression analysis, N=1,727) | Depression/anxiety, suicidal thoughts, aggressivity, obsessive-compulsive, delusions, and hallucinations | UHDRS-b | Functional capacity | UHDRS TFC (citations for Shoulson and Fahn TFC) | Hierarchical multiple regression | None of the psychiatric symptoms accounted for a statistically significant amount of variance in TFC. |
| Sheppard et al. (33) | Cross-sectional (patients with Huntington’s disease, N=20, control subjects, N=20) | Depression | BDI-II (study subjects <65 years old), GDS-short form (study subjects ≥65 years old) | Instrumental activities of daily living | Lawton and Brody Activities of Daily Living Questionnaire | Spearman’s rank correlations; Depression scores were dichotomized (elevated or nonelevated) | Depression was significantly correlated with the self-report Lawton Instrumental Activities of Daily Living Scale. |
| Sprengelmeyer et al. (28) | Cross-sectional, prospective (patients with Huntington’s disease, N=61, control subjects, N=40) | Depression | HADS-SIS | Ability to cope with demands of daily life | UHDRS TFC | Mann-Whitney test | Depressed and nondepressed Huntington’s disease groups showed no statistically significant difference in TFC scores. |
| van Duijn et al. (29) | Cross-sectional, retrospective (patients with Huntington’s disease, N=1,993) | Neuropsychiatric symptoms (predetermined five subscales: depression, irritability/aggression, obsessive-compulsive behaviors, apathy, and psychosis) | UHDRS-b (predetermined five subscales: depression, irritability/aggression, obsessive-compulsive behaviors, apathy, and psychosis) | Global functioning | UHDRS TFC | One-way between groups analysis of variance or nonparametric Kruskal-Wallis test or univariate analyses; multivariate logistic regression | Patients with Huntington’s disease with each of the five symptoms measured (depression cluster, irritability/aggression, obsessive-compulsive behaviors, apathy, and psychosis) had lower TFC scores than patients without these symptoms (p<0.001 for all), but on multiple regression, TFC scores only statistically significantly correlated with apathy. |
Results
| Symptom and neuropsychiatric tool | Functional capacity tool | Effect sizeb | r or β (rounded to the nearest hundredth)c | Study |
|---|---|---|---|---|
| Individual symptoms | ||||
| Depressive symptoms | ||||
| HAM-D | FAS | r=–0.43 | 0.43 | Banaszkiewicz et al. (2) |
| UHDRS-b | FAS | r=0.039 | 0.04 | Beglinger et al. (30) |
| PBA-s | TFC | r=–0.003 | 0.01 | Eddy and Rickards (26) |
| BDI-II | TFC | r=0.52 | 0.52 | Epping et al. (23) |
| BDI | TFC | r=–0.69 | 0.69 | Mayeux et al. (24) |
| BPRS | TFC | r=–0.76 | 0.76 | Mayeux et al. (24) |
| BDI-II | Lawton and Brody ADL | r=–0.57 | 0.57 | Sheppard et al. (33) |
| HADS-SIS | TFC | r=0.03 | 0.03 | Sprengelmeyer et al. (28) |
| Suicidality | ||||
| PBA | TFC | r=0.157 | 0.16 | Hubers et al. (31) |
| UHDRS-b | TFC | β=–0.025 | 0.03 | Nehl and Paulsen (1) |
| Anxiety | ||||
| UHDRS-b | FAS | r=–0.2 | 0.2 | Banaszkiewicz et al. (2) |
| PBA-s | TFC | r=–0.26 | 0.26 | Eddy and Rickards (26) |
| Irritability | ||||
| UHDRS-b | FAS | r=0.02 | –0.02 | Banaszkiewicz et al. (2) |
| Aggression | ||||
| UHDRS-b | FAS | r=–0.19 | 0.19 | Banaszkiewicz et al. (2) |
| UHDRS-b | TFC | β=–0.03 | 0.03 | Nehl and Paulsen (1) |
| Apathy | ||||
| UHDRS-b | FAS | r=–0.47 | 0.47 | Banaszkiewicz et al. (2) |
| PBA-s | TFC | r=–0.096 | 0.1 | Eddy and Rickards (26) |
| PBA-s | Composite of TFC, FAS, and IS | adjusted r2=0.14 | 0.37 | Fritz et al. (3) |
| FLOPS | TFC | r=0.77 | 0.77 | Hamilton et al. (32) |
| HD-ADL (instrumental) | r=0.92 | 0.92 | ||
| HD-ADL (physical) | r=0.83 | 0.83 | ||
| UHDRS-b | TFC | r=0.058 | 0.06 | van Duijn et al. (29) |
| Delusions | ||||
| UHDRS-b | TFC | β=–0.044 | 0.04 | Nehl and Paulsen (1) |
| Hallucinations | ||||
| UHDRS-b | TFC | β=–0.013 | 0.01 | Nehl and Paulsen (1) |
| Symptom clusters | ||||
| Depression cluster 1 (depression and anxiety) | ||||
| UHDRS-b | IS | β=0.061 | 0.06 | Marder et al. (27) |
| UHDRS-b | TFC | β=–0.02 | 0.02 | Nehl and Paulsen (1) |
| Depression cluster 2 | ||||
| UHDRS-b | TFC | r=0.008 | 0.01 | van Duijn et al. (29) |
| Irritability/aggression cluster | ||||
| UHDRS-b | TFC | r=0.008 | 0.01 | van Duijn et al. (29) |
| Obsessive-compulsive cluster | ||||
| UHDRS-b | TFC | r=0.096 and r=0.308d | 0.1 | Anderson et al. (25) |
| 0.31 | ||||
| UHDRS-b | TFC | B=–0.055 | 0.06 | Nehl and Paulsen (1) |
| UHDRS-b | TFC | r=0.017 | 0.02 | van Duijn et al. (29) |
| Psychosis cluster | ||||
| UHDRS-b | FAS | r=–0.25 | 0.25 | Banaszkiewicz et al. (2) |
| UHDRS-b | TFC | r=0.017 | 0.02 | van Duijn et al. (29) |
| Total behavioral score | ||||
| UHDRS-b | FAS | r=–0.35 | 0.35 | Banaszkiewicz et al. (2) |
Measurement of Functional Capacity
| Measure | Validity | Reliability |
|---|---|---|
| Shoulson and Fahn TFC (11, 12) | Concurrent validity: measures of caudate atrophy on MRI and metabolism on positron emission tomography significantly correlated with TFC scores (p values for both <0.001). Face validity: created by eight field experts, widely used. | Interrater reliability for agreement within 1 point was 65%, and within 2 points it was 85% (48). |
| UHDRS TFC (20) | Face validity: widely used in Huntington’s disease clinical trials. Concurrent validity: significantly correlated with caudate change (49). Convergent validity: significantly intercorrelated with IS (0.86) and FAS (0.9) (p<0.001) (50). | No Cronbach’s alpha or interrater reliability was reported for this subscale, although HSG (1996) cites reliability data for the Shoulson and Fahn TFC scale. It was highly intercorrelated with UHDRS motor, behavioral, and functional checklist scores (p<0.005), which had Cronbach’s alpha values of 0.95, 0.9, and 0.95, respectively. |
| UHDRS FAS (20) | Convergent validity: intercorrelations with a TFC score of 0.9 and an IS score of 0.91 (p values for both <0.001) (50). | High internal consistency: Cronbach’s alpha was 0.95. |
| UHDRS IS (20) | Convergent validity: intercorrelations with a TFC score of 0.86 and an FAS score of 0.91 (p values for both <0.001) (50). | No Cronbach’s alpha or interrater reliability was reported for this subscale. It was highly intercorrelated with UHDRS motor, behavioral, and functional checklist scores (p<0.005), which had Cronbach’s alpha values of 0.95, 0.9, and 0.95 respectively. |
| Lawton and Brody Activities of Daily Living questionnaire (21) | Not established in Huntington’s disease diagnosis (developed for older adults); used in one Huntington’s disease study. | Interrater reliability showed a correlation of 0.85 between total instrumental ADL scores. |
| HD-ADL (22) | Convergent validity: correlates with Shoulson and Fahn TFC (r=–0.89). Construct validity: PCA identified four factors accounting for 74% of variance (all eigenvalues >1). | High internal consistency: Cronbach’s alpha was 0.91. |
Relationship of Total Neuropsychiatric Symptoms With Functional Capacity
Relationship of Individual Neuropsychiatric Symptoms With Functional Capacity
Depression.
| Measure | Validity | Reliability | Considerations |
|---|---|---|---|
| UHDRS-b (20) | Construct validity: factor analyses has shown heterogenous factors (19, 42). Convergent validity: shown between the depression item on the UHDRS-b and the “feel sad” item on the BDI (correlation coefficient=0.834, p<0.01), as well as the “depressed mood” item on the HAM-D (r=0.917) (41). Face validity: the longest-used tool for behavioral symptoms in Huntington’s disease. | Internal consistency: Cronbach’s alpha was equal to 0.83. | Although the whole tool has established validity and reliability, single items are often used in isolation to assess only one symptom. Depression has established convergent validity with the BDI and HAM-D, but no other individual item has established validity, and no individual item has reliability data to support its use. |
| HAM-D (34) | Convergent validity: validity with UHDRS-b “depressed mood” item (r=0.917) (41). Face validity: used to measure depression in several Huntington’s disease studies. | Interrater reliability was equal to 0.9. | Used to assess depressive symptoms only. |
| PBA (40) | Content/face validity: created by a panel of Huntington’s disease experts from a list of patient symptoms and complaints and extensive literature review. Construct validity: PCA identified three factors, although these factors only accounted for 40.7% of the total variance. | Interrater reliability was >0.8, and the test-retest reliability was >0.9 for the whole scale. Depression cluster items showed good internal consistency (Cronbach’s alpha=0.81) (42). | Although the whole tool has established validity and reliability, single items are often used in isolation to assess only one symptom. Depression cluster items have established reliability data, but individual symptoms do not. |
| BDI-II (36) | Criterion validity: diagnosis of depression (compared with the study gold-standard Schedules for Clinical Assessment in Neuropsychiatry) at a score of 10 or 11 had a sensitivity of 1.0, specificity of 0.66, and area under the curve of 0.856 (51). | Internal consistency in outpatients was high (Cronbach’s alpha=0.92) (52). | Used to assess depressive symptoms only. |
| PBA-s (18) | Construct validity: factor analysis (after excluding paranoid thinking and hallucinations as a result of low incidence) revealed three factors (apathy, irritability, and affective) with eigenvalues >1, consistent with the factor structure in the PBA-HD. Face validity: used in several large clinical or observational trials. | Interrater reliability was considered decent for severity (0.74, adjusted=0.77) and frequency (0.76, adjusted=0.8). No reliability data for individual symptoms were available. | Although the whole tool has established validity and reliability, single items are often used in isolation to assess only one symptom. No individual symptom item has its own established reliability. |
| BDI (35) | Content validity: addresses most of DSM-III criteria for depression and intentionally excluded other criteria due to the frequency of their presence in nondepressed patients (53). Convergent validity: the “feel sad” item correlates with the UHDRS “depressed mood” item (r=0.834) (41). The mean correlation coefficient with HAM-D was 0.73 (53). | Internal consistency: the mean coefficient alpha for psychiatric patients was 0.86, and for nonpsychiatric patients it was 0.81 (53). Interrater agreement between patients with Huntington’s disease and their caregivers was moderate to good (54). | Used to assess depressive symptoms only. |
| BPRS (37) | Interrater reliability for depressive mood was 0.82. | In this review, it was used only to measure depressive symptoms. Depressive mood items have decent reliability data, but there is no real validity data for its use in Huntington’s disease. | |
| HADS-SIS (38) | Convergent validity: correlation between the modified HAM-D and depression items of the HADS-SIS was equal to 0.75 (p<0.05). | Internal consistency of the depression subscale Spearman-Brown coefficients ranged between 0.72 and 0.81. | Validity and reliability data are available for the depression subscale (the only one used in this review), but data are weaker compared with other scales. |
| GDS-short form (39) | Convergent validity with BDI in preoperative surgical patients (Spearman’s r=0.704, p<0.01) (55). | Internal consistency: Cronbach’s alpha was 0.749 (in adults >64 years old) (56). | No true validity data for Huntington’s disease are available, and because it was created for older adults, this makes validity in a younger Huntington’s disease population even more questionable. |
| FLOPS (57) | Construct validity: used in measuring frontal lobe symptoms in patients with frontal lobe lesions versus the same patients before they had lesions, as well as healthy control subjects (p<0.001). Factor analysis revealed that 83% of items were loaded on three factors (58). Face validity: patients with Huntington’s disease were included in the factor analysis (58). | Internal consistency: Cronbach’s alpha was 0.95. | Not clearly valid in Huntington’s disease, although patients with Huntington’s disease were included in the sample for factor analysis (58). |
Suicidality.
Anxiety.
Irritability.
Aggression.
Apathy.
Delusions.
Hallucinations.
Obsessions, compulsions, perseveration.
Relationship of Neuropsychiatric Symptom Clusters With Functional Capacity
Depression clusters.
Irritability–aggression cluster.
Obsessive-compulsive cluster.
Psychosis cluster.
Other.
Discussion
Conclusions
Acknowledgments
References
Information & Authors
Information
Published In
History
Keywords
Authors
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

