Approximately 5%–20% of those in nontreatment samples (i.e., individuals not seeking treatment) of U.S. troops returning from Iraq and Afghanistan have posttraumatic stress disorder (PTSD) (
1,
2). More than 400,000 service members were treated for PTSD at Veterans Administration (VA) facilities between 2002 and 2015 (
3). A signature weapon of the present conflicts in the Middle East has been the improvised explosive device, which can cause concussive injuries. Consequently, in addition to the psychological consequences of combat exposure, an estimated 11%–23% of combatants return with traumatic brain injury (TBI) (
4). From 2001 to 2013, more than 200,000 cases of TBI were recorded in the VA Health Registry; of these, 84% were service related and associated with ongoing symptomatology; 81% of the 200,000 were classified as mild (
5).
Both PTSD and mild TBI (mTBI) are associated with some degree of neurocognitive limitation (
6), although with somewhat differing profiles. PTSD has been associated with limitations in initial stimulus encoding, sustained attention, acquisition, retrieval of new verbal information, and executive functions (
7–
9). However, in the absence of prospective studies, it may be impossible to rule out the possibility that at least some of these limitations may have predated both the trauma and PTSD (
10). There are also a wide range of potential confounding factors, including direct CNS effects associated with the trauma (e.g., torture and toxic exposures [
11]), patterns of posttrauma substance use (
12), the presence of comorbid psychiatric conditions (
13), and incentive to perform poorly on testing for financial and other reasons (
14–
16).
Acute mTBI is associated with slowed cognition, attentional limitations, defects in new verbal and visual learning, impaired oral fluency, and executive function limitations (
17,
18). Research suggests that objective indices of cognitive limitation after mTBI tend to normalize by about 3 months postinjury (
19), although this view has been recently challenged (
20).
An additive effect of comorbid PTSD and TBI is suggested by findings of more extensive self-reported and objective cognitive limitations (processing speed and executive functioning) among veterans with both conditions compared with those with mild TBI alone (
21). Given the high rates of PTSD and mTBI among returning service members, as well as the high rate of their co-occurrence, it is important to understand how mTBI may affect the treatment of PTSD.
Currently, cognitive-behavioral therapies (CBTs)—specifically, prolonged exposure therapy (
22) and cognitive-processing therapy (
23)—are the first choices of evidence-based treatments for PTSD (
24). However, it is unclear whether individuals with cognitive limitations related to either PTSD or mild TBI are able to benefit from treatments that rely, at least in part, on cognition. Some investigators have found that poorer cognitive performance (verbal memory) is associated with poorer response to CBT for PTSD (
25–
27). Poorer verbal learning has also been associated with poorer response to treatment for PTSD nightmares (
28). Alternatively, cognitive limitations may affect adherence to, but not benefit from, treatment among individuals who complete treatment. For example, in women who have PTSD as a result of sexual assault, lower intelligence scores (although within the normal range) and lower education have been associated with higher CBT dropout but not with less treatment efficacy among those who complete treatment (
29). Similarly, among patients with PTSD and comorbid schizophrenia, schizoaffective disorder, major depression, or bipolar disorder, poorer cognitive performance (on a composite measure including attention, information processing speed, verbal learning and memory, and executive functioning) predicted poorer learning of information about posttraumatic stress symptoms but not clinical benefit from CBT (
30).
The influence of TBI on the efficacy of CBT among individuals with PTSD has not been systematically examined. Two studies examined the effectiveness of cognitive processing therapy among veterans with PTSD and comorbid TBI in a residential treatment setting. After 7 weeks of treatment, participants had pretreatment-to-posttreatment reductions in both PTSD scores (
31,
32) and postconcussive symptoms (
32), and these reductions were positively correlated (
32). However, the intervention included cognitive rehabilitation, which might have confounded the association between CBT and change in PTSD symptoms. In addition, cognitive limitations were measured by self-report rather than objective tests, which diminishes the validity of the results. A third small study of veterans with PTSD and TBI of mild (N=6) and moderate (N=4) severity found reduction in PTSD symptoms of nearly 50% after a course of prolonged exposure therapy. Both the mild and moderate TBI severity groups improved from pre- to posttreatment (
33). The study included objective cognitive limitation measurements, but they were not part of the published analyses. However, it cannot be assumed that TBI necessarily equates with cognitive limitations. None of the aforementioned studies included the latter as an independent (predictor) variable.
Thus, the literature on cognitive predictors of treatment response to CBT for PTSD remains insufficient and inconclusive because studies are scant, study populations differ, treatment models are difficult to compare, and few studies include objective measures of cognition. In the present study, we examined whether objectively measured pretreatment cognitive performance would predict PTSD treatment outcome in veterans and active-duty military service members receiving prolonged exposure or cognitive-processing therapy in a naturalistic outpatient setting. We hypothesized that poorer pretreatment cognitive ability (new learning, memory, processing speed, complex attention, inhibition, and flexibility) would be associated with poorer treatment response to CBT for PTSD.
Methods
Participants
Participants were recruited from the Massachusetts General Hospital Home Base Program, a private-public partnership between Massachusetts General Hospital and the Red Sox Foundation whose mission is to serve the clinical needs of Operation Enduring Freedom, Operation Iraqi Freedom, and Operation New Dawn (OEF/OIF/OND) active-duty service members, reservists, and veterans. As part of its treatment as usual, the Home Base Program provides manualized individual CBT (prolonged exposure therapy or cognitive-processing therapy) for patients with PTSD. Participants included in the final analysis (N=23; male, N=20, female, N=3) were a convenience sample of OEF/OIF/OND veterans and active-duty service members between the ages of 18 and 50 years who met DSM-5 criteria for chronic (more than 1 year posttrauma) combat-related PTSD. Participants were classified as having PTSD only (i.e., no history of TBI) or PTSD plus mTBI. In addition to the same inclusion criteria for individuals in the PTSD-only group, participants in the PTSD plus mTBI group had to have a diagnosis of mTBI as determined by the VA/DoD Clinical Practice Guideline for Management of Concussion/Mild Traumatic Brain Injury (
34); an mTBI that occurred ≥12 months before study entry (to minimize natural recovery from TBI that could confound treatment outcome); current postconcussive symptoms, with a score ≥20 on the Neurobehavioral Symptom Inventory; and current cognitive complaints, with a score of 3 (severe) on at least one of three items on the Neurobehavioral Symptoms Inventory (“poor concentration, can’t pay attention, easily distracted”; “forgetfulness, can’t remember things”; or “slowed thinking, difficulty getting organized, can’t finish things”). Because patients with mTBI frequently recover to their baseline cognitive function, this additional requirement was designed to identify patients with TBI who continued to experience cognitive symptoms at the time of the treatment intervention, which, we hypothesized, may be a pivotal factor interfering with successful outcome from CBT among such patients. Patients who were identified as eligible were approached by their therapist about their interest in participating.
Exclusion criteria were greater than mild TBI (as defined in the VA/DoD Clinical Practice Guideline) (
34); a history of neurological disorder (e.g., stroke, epilepsy, multiple sclerosis, HIV, or neurodegenerative disorder); an acute or unstable medical condition likely to impair cognition or interfere with participation in the study; current risk of suicide (as determined by the Concise Health Risk Tracking scale, Self-Report (
35); current psychotic disorder or melancholia; current or lifetime history of bipolar disorder; inability to attend regular appointments; prior intolerance or failed adequate trial of CBT; use of a psychostimulant (including modafinil); use of skeletal muscle relaxants, narcotics, anticonvulsants, neuroleptics, or any other medication that could impair cognition or interfere with the assessments, as determined by usage history or urine testing; and a medication dosage that would likely change during the study time period.
The PTSD-only group comprised seven male veterans, and the PTSD plus mild TBI group comprised 13 male veterans and three female veterans. Other characteristics appeared to be similar between the two treatment groups.
Procedure
Patients were screened for eligibility during their regular evaluation process at the Massachusetts General Hospital Home Base Program. Individuals who appeared to satisfy the inclusion criteria were considered for potential participation. After full explanation of the study procedures, written informed consent was obtained. Participants received a baseline screening that included the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5) and the Structured Clinical Interview for DSM-IV Axis I Disorders. Cognitive measures were completed at baseline assessment, at CBT completion, and at 6 months after treatment completion. Participants completed the PTSD Checklist for DSM-5 (PCL-5) at baseline, at each weekly CBT session, at 1 month after CBT completion, and again at 6 months after CBT completion. In addition to baseline, the CAPS-5 was administered after every four sessions of CBT, 1 month after CBT completion, and 6 months after CBT completion. Participants received a small remuneration for their time spent in the study assessments. There were 12 planned manualized sessions of either prolonged exposure therapy or cognitive-processing therapy, according to therapist judgment and patient choice. Participants who received prolonged exposure therapy were instructed about the nature of PTSD with an emphasis on the role of avoidance behaviors that serve to maintain the disorder. Prolonged exposure therapy consisted of breathing retraining, in vivo exposure to feared situations and places, and imaginal exposure to the trauma memory. Participants who received cognitive processing therapy were instructed about the nature of PTSD with an emphasis on the role of maladaptive cognitions (i.e., “stuck points”) that serve to maintain the disorder. During cognitive-processing therapy, participants learned about the connection between thoughts and feelings in order to identify problematic patterns of thinking and to effectively question assimilated (e.g., “It’s my fault”) and accommodated (e.g., “Authority cannot be trusted”) assumptions regarding safety, trust, intimacy, power, control, and self-esteem. Therapists were blind to the baseline neurocognitive assessment. This study was approved by the institutional review board of Partners Health Care System.
Measures
PCL-5 total scores served as the primary treatment outcome measure (
36). The PCL-5 is a validated 20-item self-report assessment of PTSD severity, with good internal consistency (α=0.96), good test-retest reliability (r=0.84), and good convergent and discriminant validity (
37). Internal consistency for the present study was very good (standardized Cronbach’s α=0.77). Additionally, an experienced psychometrician administered the gold standard for evaluating PTSD: the CAPS-5 (
38). The CAPS-5 internal consistency for the present study was very good (standardized Cronbach’s α=0.81).
Our nine predictor measures, assessed at pretreatment baseline, encompassed several cognitive domains important to PTSD and consisted of the following:
1.
Rey Auditory Verbal Learning Test (RAVLT) (
39), a measure of new learning and verbal memory, sum of scores for trials 1–5 (RAVLT
1–5);
Z scores from published normative samples (age-adjusted) (
40) were used.
2.
RAVLT delayed recall after distraction score (RAVLT trial 7);
Z scores from published normative samples (age-adjusted) (
40) were used.
3.
Wechsler Adult Intelligence Scale, Fourth Edition (WAIS-IV) (
41) letter-number sequencing subtest (scaled score, age-adjusted), a measure of complex attention and working memory.
4.
WAIS-IV coding subtest (scaled score, age-adjusted), a measure of cognitive processing speed.
5.
Delis-Kaplan Executive Function System (D-KEFS) (
42), a measure of inhibition and cognitive flexibility, color-word inhibition (scaled score, age-adjusted).
6.
D-KEFS inhibition/switching (scaled score, age-adjusted).
7.
D-KEFS letter fluency (scaled score, age-adjusted), which measures verbal fluency.
8.
D-KEFS category fluency (scaled score, age-adjusted), which measures verbal fluency.
9.
Advanced Clinical Solutions Test of Premorbid Functioning (
41) standard score (age-adjusted), a measure of premorbid intellectual function.
In addition, we used the Test of Memory Malingering (TOMM), a visual recognition test sensitive to reduced effort or malingering but insensitive to neurological impairments (
43). A TOMM trial 1 score ≥41 has been shown to be a useful indicator of adequacy of effort among veterans (
44), and thus administration of trials 2 and 3 may be unnecessary in this population.
Participants were classified as having mTBI if a history of TBI was identified through review of clinical records or through a structured interview (
45) and if they had a score of 3 (severe) on at least one of the following three Neurobehavioral Symptom Inventory cognition items: “poor concentration, can’t pay attention, easily distracted”; “forgetfulness, can’t remember things”; or “slowed thinking, difficulty getting organized, can’t finish things.” Because some patients with mTBI recover to their baseline cognitive function, the additional requirement of scoring “severe” on at least one cognition item of the Neurobehavioral Symptom Inventory was designed to identify participants with TBI who continued to experience cognitive symptoms at the time of the treatment intervention. We hypothesized that such cognitive symptoms might interfere with their ability to benefit from CBT.
Data Analysis
Linear regression was used to regress the slope of PCL-5 scores on the time elapsed (weeks) after informed consent, separately for each participant. Next, individual PCL-5 slopes were correlated with cognitive scores measured at the baseline assessment, separately. The same two-step analysis was used for CAPS-5 scores. Because a negative slope indicates a progressive reduction of PTSD symptoms (i.e., improvement) and higher baseline cognitive testing scores indicate better cognitive functioning, the hypothesis that poorer cognitive functioning would be associated with poorer treatment response is supported by a negative correlation. Additionally, individual changes in scores on the PCL-5 and CAPS-5 (ΔPCL-5 and ΔCAPS-5, respectively, i.e., posttreatment minus pretreatment) were correlated with baseline cognitive scores. Because poorer treatment response indicates less change in score, again, this hypothesis is also supported by a negative correlation. No data imputation was performed. All analyses were conducted with the SAS 9.4 software package (SAS, Cary, N.C.).
Results
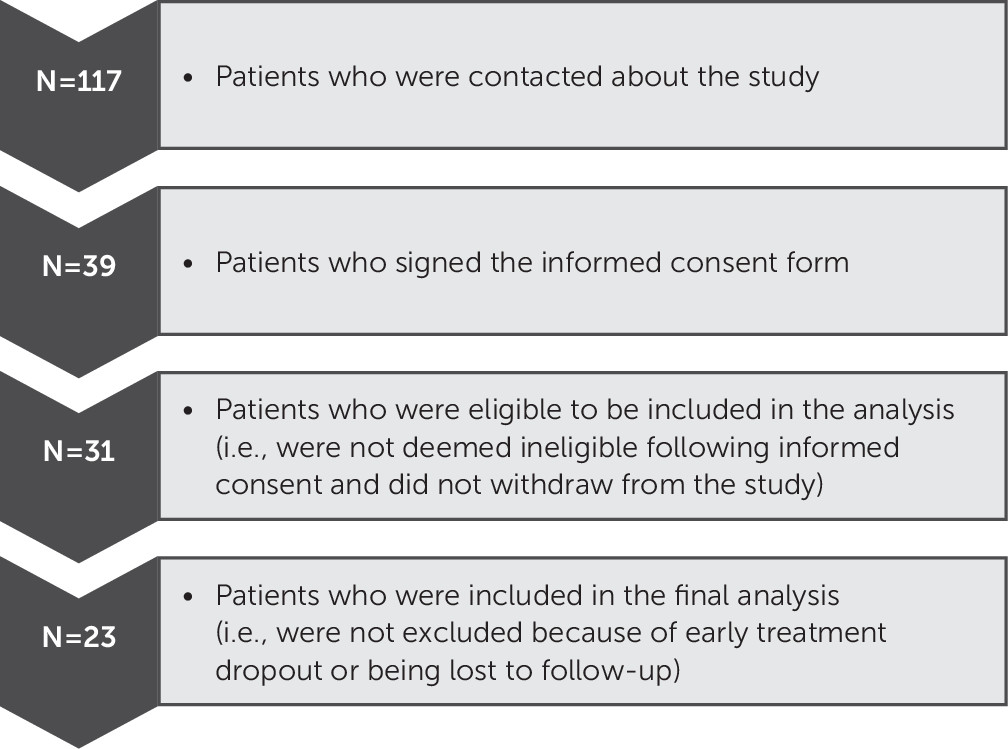
Participant recruitment within our naturalistic design is presented in
Figure 1. Demographic data for the study participants are presented in
Table 1. Briefly, the majority of participants were male (87.0%), white (82.6%), and single (47.8%), and the mean age was 32.4 years (SD=6.0). Nearly seventy percent (69.6%) of participants had comorbid PTSD and mTBI. Most participants (73.9%) received prolonged exposure therapy. The means and standard deviations for our baseline predictor measures and pretreatment and posttreatment outcome measures are presented in
Table 2. It is noteworthy that PCL-5 scores decreased by an average of 26 points, and CAPS scores decreased by an average of 14 points, indicating substantial but incomplete improvement. Cognitive score means were generally within the normal range, but there was good variability in cognitive test scores and clinical outcome measures. Correlations of baseline cognitive scores with PCL-5 and CAPS-5 individual slopes are presented in the top half of
Table 3; those of baseline cognitive scores with ΔPCL-5 and ΔCAPS-5 are presented in the bottom half of
Table 3. D-KEFS color-word inhibition positively correlated with the PCL-5 individual slope but not with the CAPS-5 slope, ΔPCL-5, or ΔCAPS-5. No other significant correlations were found between baseline cognitive scores and individual PCL-5 or CAPS-5 slopes, ΔPCL-5, or ΔCAPS-5. Accounting for the effects of age, education, ethnicity, and type of therapy (cognitive-processing therapy compared with prolonged exposure therapy) by using each of them separately in partial correlations did not change the above correlations. All except one participant showed good effort on our validity measure (TOMM trial 1): of 23 veterans, only one received a score <41 (with a score of 29). Removing this participant’s data did not substantially change the results. Therefore, we included all 23 participants in the final analyses.
In order to address the issue of type II error in the face of negative results, we averaged the lower (predicted direction) confidence limits of the correlations between each of the nine cognitive predictors and the treatment outcome measures (PCL slope, –0.33; ΔPCL-5, –0.44; CAPS slope, –0.58, and ΔCAPS, –0.41). Cohen has proposed the following descriptors for effect sizes: r=0.1 mild, r=0.3 moderate, and r=0.5 strong (
46,
47).
Discussion
We investigated the effect of pretreatment cognitive limitations on PTSD outcomes among veterans receiving evidence-based psychotherapies (i.e., prolonged-exposure therapy or cognitive processing therapy) in a naturalistic treatment setting. Contrary to our prediction, pretreatment cognitive limitations did not significantly predict poorer PTSD symptom response to CBT. Inspection of the correlations table (
Table 3) did not reveal any meaningful patterns. For example, the only statistically significant finding was that D-KEFS color-word inhibition positively correlated with individual PCL-5 slope, thereby militating against the hypothesis. However, similar correlations were not evident with the other outcome measures (CAPS-5 slope, ΔPCL-5, and ΔCAPS-5). There was notable lack of consistent patterns for the remainder of the cognitive baseline variables. Additionally, we found no evidence that the presence of categorical mTBI correlates with poorer outcomes with CBT. Examination of confidence limits for the PCL-5 outcome measure supports the conclusion that these results do not represent a type II error with regard to the absence of a strong association, but they do not refute the possibility of a medium association.
Our findings are consistent with studies that found no association between cognition and CBT outcome (
30) and no association between the presence of mild TBI and the ability to benefit from CBT for PTSD (
31–
33). The similarity of our results to those in these studies may be partially a result of comparability of study populations (OIF/OEF/OND veterans) and treatment approaches (
31–
33). On the other hand, our findings differ from studies that found an association between cognition and CBT outcome (
25–
28). These differences may be partially a result of differences in study samples (veterans in our study versus civilians in the studies by Wild and Gur [
25] and Nijdam et al. [
27] and predominantly male veterans in our study versus only female veterans in the Haaland et al. [
26] study) and differences in outcomes (e.g., PTSD symptoms in our study versus nightmare distress and severity in a previous study [
28]).
This study has several limitations. As noted above, results from a larger sample could have conveyed greater protection against type II error. Additionally, our naturalistic treatment design did not allow us to rule out the contributions of multiple unmeasured factors (e.g., socioeconomic status and substance use) that may have confounded the true association between cognitive limitations and change in PTSD symptoms following CBT. The study design did not incorporate a means for determining whether the objective cognitive limitations for any given participant in the PTSD plus mTBI group were a result of mTBI or other factors (which is not an easy task). Given these limitations, independent replication of our results with larger samples is warranted.
In contrast, our naturalistic design increases the generalizability of our results to other real-world PTSD populations encountered in veteran outpatient clinics. An additional strength of our study is that our main predictor construct (cognition) was measured by means of objective and validated cognitive measures rather than mere self-report.
The major clinical implication of this study is that—other things being equal—individuals with poorer neurocognitive abilities should not be assumed to benefit less from CBT for PTSD than those without such limitations. Our results discourage any notion of excluding PTSD patients with poorer cognitive abilities from receiving CBT.