Functional neurological disorder (FND) is a ubiquitous disorder, with severity of disability often exceeding that of comparable neurological illnesses (
1,
2). Recent imaging studies of FND suggest that disconnections between the hypoactive prefrontal and supplemental motor cortex, as well as hyperactive limbic activity, may be responsible in part for the motor-sensory symptoms (
3–
6). These identified areas may be possible targets of engagement for treatment innovations and correspond to correlates of current interventions, such as mirror visual feedback (MVF) (
7,
8) and exposure therapy (ET) (
9); however, neither has been tested in FND. MVF and ET are used in many disorders that are similar to and comorbid with FND, such as complex regional pain syndrome (CRPS) and posttraumatic stress disorder (PTSD) (
10–
13). Both MVF and ET treatments are now more available and amenable to standardized delivery using immersive technology and commercially available virtual reality (VR) devices.
MVF, although originally reported as a treatment for phantom limb pain, has been used in multiple unilateral pain-disuse syndromes, such as CRPS type I, and upper-extremity motor recovery, such as poststroke hemiparesis (
13). MVF involves creating a visual limb-swapping illusion of an affected limb moving normally. Perceived movement is controlled by a contralateral unaffected limb. Lack of movement or abnormal movements are replaced by the visual perception of normal movement, which increases visual and somatosensory areas of the brain to resolve incongruence and enhances monitoring. This has been documented to lead to increases in attention and cognitive control. In addition, MVF has been shown to increase excitability of the ipsilateral primary motor cortex that projects to affected body parts, leading to increased action control (
8). Neuroimaging and clinical experience also suggest that cortical reorganization after this intervention may be long lasting (
14). To date, no exploratory studies of MVF for FND have been reported, despite the frequent occurrence of unilateral motor and sensory symptoms in FND. Likewise, many FND patients have comorbid pain-disuse syndromes in which MVF is being used as an augmentation to treatment (
13).
ET is a treatment technique within cognitive-behavioral therapy that involves repeated real, visualized, or simulated exposures to a feared situation or object to achieve desensitization and a decrease in maladaptive avoidance behaviors. It is considered the standard first-line treatment for most anxiety disorders (
12). ET and fear extinction are associated with normalization of altered responsivity in networks of hyperactive limbic and prefrontal-cortical brain regions, most notably amygdala activity (
9). Amygdala sensitization and abnormal habituation have been documented in FND patients and with other anxiety disorders that are comorbid with FND, such as PTSD (
3,
10). Despite ET targeting one of FND’s known abnormalities, to date no exploratory studies of formal ET for FND have been performed, except for one controlled trial of paradoxical intention (
15).
ET and MVF delivered through VR may allow more specific, standardized, and personalized treatment than traditional physical therapy or ET, which may be particularly helpful for the heterogeneous presentations encountered in FND (
16–
19). Because both VR treatments described are untested and would be somewhat resource intensive to investigate alone, exploring both simultaneously was most practical. We developed a flexible in-office MVF and ET system that used VR through two commercial gaming headsets based on the work of Won et al. (
18). This article describes the preliminary midpoint findings of this proof-of-concept pilot of VR-MVF and VR-ET for FND.
Methods
Participants
Following approval from Stanford University’s Human Subject’s Research and Institutional Review Board and clinical trials registration, adult patients ages ≥18 who met DSM-5 criteria for FND with any type of episodic or continuous motor or sensory symptom were recruited from Stanford Hospital’s outpatient neurobehavioral clinic between November 2016 and August 2018. Confirmation of diagnosis was required by at least two board-certified neurologists. Exclusion criteria included primary psychotic disorder, current self-harm or suicidality, change in medication or treatment in past month, pregnancy, and substance use disorder. Although comorbid general medical conditions were allowed, comorbid epilepsy was excluded because of potential safety concerns for flashing-light sensitivities. Participants were randomly assigned to receive either eight sessions of VR-MVF or to a control arm, in a single-blind fashion by using permuted-blocks of five in sealed envelopes. A target sample size of 30 patients was selected in keeping with the higher end of the range of sample sizes used for such feasibility studies.
Design
A single-blind placebo-controlled trial was designed. In a single psychiatry office, all participants received eight sessions, each 5–20 minutes long, of a body-tracking VR experience delivered by a commercially available HTC-VIVE gaming headset. All participants experienced a 360-degree landscape from an egocentric viewpoint, which included trees and that could be explored by walking in an office play space. Time in the 6-degrees-of-freedom environment was dictated by the participant’s tolerance and desire to stay in the environment but never exceeded 20 minutes. All interventions were performed by unblinded providers.
Treatment Arm
In the treatment arm, VR-MVF (
Figure 1A) was delivered in each session. Participants visually inhabited and embodied an entire or partial avatar body that included an affected limb involved in at least one of their sensory-motor symptoms. The contralateral asymptomatic limb movements were programmed to be swapped for their affected limb movements prior to each session. Participants explored, at their leisure, a world and body in which they could pop balloons, stack stones or blocks, look into a mirror, or walk in a 360-degree landscape and explore a nearby tree or lake.
VR-ET was delivered weekly starting at session 4 and beyond and after VR-MVF. During the session, participants collaborated with the provider in a search of YouTube VR-360 video channel content on their own mobile phones and were asked to identify personalized typical FND symptom triggers. If content was available, participants were invited to engage in a three-degrees-of-freedom VR experience by using Google Cardboard for 1–10 minutes, based on each person’s tolerance and desire to continue and not exceeding a maximum subjective units of distress (SUDS) of 8/10 or 10 minutes of use. Participants were given Google Cardboard to take home and instructed to participate in these exercises as they desired at home with their own mobile devices at least once per day but not to exceed a SUDS rating of 7 at any time during the exercise.
Control Arm
Participants in the control arm received a similar but nonembodied commercially available HTC-VIVE experience on STEAM VR using an application called LUMEN (
Figure 1B) that did not involve hand controls. The immersive experience consisted of participants’ visual gaze causing objects, such as trees and flowers, in the environment to grow. Participants were able to freely move within the 360-degree play space. At study end, participants were informed that they had received the sham treatment and were invited to receive the experimental treatment if they desired.
Measures
Demographic characteristics and clinical history were collected by written survey forms during enrollment and by a formal 1.5-hour psychiatric interview by the principal investigator (K.B.). Feasibility was measured by adverse events, dropouts, screen failures, side effects, retention, and integrity of the blinding procedures and by qualitative feedback on exit interview. The primary and secondary outcome measures were symptom frequency and the Oxford Handicap Scale (OHS) (
20), respectively. Symptom frequency was measured prospectively by the participant’s self-report in 1-week intervals, using a calendar described in the study by LaFrance et al. (
21) for psychogenic nonepileptic seizures and modified for FND. OHS was used as a secondary measure of disability to benchmark the results to three previous FND randomized controlled trials that used this measure (
21–
23). OHS is a grading scale providing information about disability; scores range from 0, no disability, to 5, severe disability. This measure was assessed by the unblinded principal investigator.
A descriptive and graphical data comparison of pre and post primary and secondary outcome measures was conducted at study midpoint. Quantitative statistical analysis was postponed until completion of the study to avoid increasing unblinded rater bias before study completion. Final analysis will include estimated change over time and will use standard linear mixed-effects modeling, with all available cases and intention-to-treat principles.
Results
Feasibility
Fifteen participants were recruited and screened, and 14 individuals between the ages of 22 and 71 agreed to randomization (see CONSORT diagram in the online supplement). The clinical characteristics of the cohort thus far are described in tables in the online supplement. Four participants were male. Twelve were of Caucasian descent and two of Asian descent. Comorbidities included PTSD, autism spectrum disorder, traumatic brain injury, and Meniere’s disease. No study participant had solely unilateral symptoms. One participant experienced continuous symptoms. Ten had known reproducible triggers for their FND symptoms, ranging from football stadiums to narrow hallways. Eight participants were receiving disability income.
One enrolled participant declined random assignment because of symptom improvement. Fourteen remaining participants were randomly assigned, with seven allocated to the treatment arm and seven to the control arm. Two early dropouts occurred in the treatment arm, for a completion rate of 86% (12/14). One participant was dropped from the study at session 5, because she met the exclusion criterion of pregnancy and risk of fall was a concern. Another treatment arm participant dropped out at session 7 because of financial problems requiring her to move out of the area. No dropouts occurred in the control arm.
Design acceptability.
Two persons in the control arm declined the optional experimental treatment at study end out of disinterest. Qualitative feedback was positive and acceptability was high on exit interview for both arms as reported by subjects and providers. Participants in both the treatment and the control arms remarked that the VR experience was relaxing and engaging. No SUDS ratings above 7 were noted for participants engaging in VR-ET. One person with known FND triggers was unable to find an appropriate simulated trigger on the YouTube VR-360 video channel. The trigger involved an interpersonal transaction of money—i.e., paying a plumber for services. Blinding success was assessed on the last study visit; two of the seven participants in the treatment arm and three in the control arm incorrectly guessed their assignment.
Safety.
A single nonreportable adverse event occurred in session 3 for a control arm participant, who 1experienced a 1-week episode of increased symptom severity attributed to bright lights seen in the VR experience; however, the symptom remitted by session 5. No episodes of cybersickness occurred, as measured by Simulator Sickness Questionnaire or by symptom report.
Efficacy
Symptom severity.
The mean percentage change in symptoms for each group is shown in a figure in the online supplement. Comparisons between groups were not statistically analyzed. Overall mean symptom frequency increased in the treatment arm in session 2 and session 7, with relative improvement by the final session. Those in the control arm showed decreases in mean symptom frequency mid-study and a return to near-baseline symptom level by study end.
Disability.
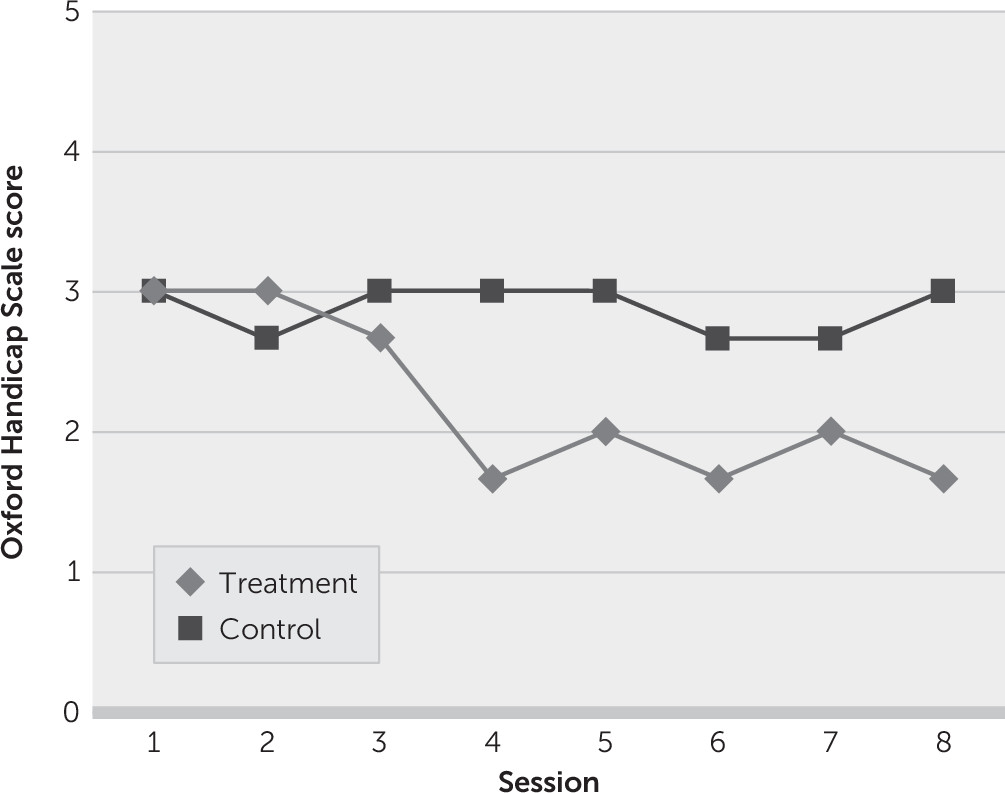
The mean OHS scores were similar at baseline for both groups (
Figure 2). Mean improvement in functioning appeared in the treatment arm at session 4 and beyond. Neither the mean symptom frequency nor disability changes between groups was statistically analyzed or calculated, and only descriptive data are presented.
Discussion
This is the first study, to our knowledge, on the use of either VR or MVF for the treatment of FND. The midpoint preliminary pilot data demonstrate adequate feasibility of this intervention as measured by high retention and completion rates, absence of adverse events, integrity of the blinding procedures, and positive qualitative feedback from participants and providers.
The limitations of this pilot study are many. The study was not powered to determine efficacy, it lacked blinded raters, relied on self-report measures, and did not assess comorbidities by using formal comprehensive interviews or scales. The statistical significance of the presented findings is unclear until study end.
On both efficacy outcome measures, there appeared to be a delay in response to the intervention of several weeks, which may have several explanations if it is later found to be statistically significant. Symptomatic gains with MVF in other clinical groups usually do not maximally occur until after several sessions (
7). It is possible that only the VR-ET was the active component of treatment. Finally, results may be evidence of an extinction pattern of behavioral learning, with initial behavioral worsening prior to improvement (
24–
26).
These preliminary results demonstrate the feasibility of using commercial VR gaming devices in an office setting to address FND symptoms. It is hoped that on the basis of this and future studies, new motor-sensory treatment options will become available that may enhance, augment, or replace existing treatments and treat comorbidities.