Major depressive disorder (MDD) in epilepsy is consistently underdiagnosed and undertreated (
1), yet it is well known that the presence of this comorbidity can lead to worse seizure outcome following treatment with medication (
2) or surgical resection (
3,
4). Novel research has conceptualized both focal epilepsy and depression as network disorders, suggesting that the substrates in each of these two disorders may be distributed in overlapping networks rather than confined to single brain regions (
5–
7). In neuroimaging and EEG studies, dysfunction in limbic temporofrontal and parietofrontal networks is consistently observed in both disorders independently (
7–
9). To our knowledge, there are no studies to date that have examined the neurophysiological signatures of network dysfunction in affective disorders in patients with epilepsy. Given the chronic, persistent nature of the symptoms of mood disorders, we hypothesized that these symptoms would likely be mediated by interictal dysfunction rather than the relatively transient neural changes that occur during seizures. Thus, we sought to determine whether there are differences in network activity during interictal periods that can distinguish patients with focal epilepsy who have self-reported symptoms of depression from patients without depression. Such studies may begin to provide a better understanding of the etiology of comorbid MDD and could lead to the development of novel personalized therapies.
Intracranial EEG (iEEG) captured during the presurgical recording period in epilepsy patients with treatment-refractory symptoms offers a particularly promising method to study MDD networks in epilepsy. This technique allows for both high temporal resolution and spatial precision and enables direct neural recordings across cortical and deep structures.
In this pilot study, we examined four regions across a corticolimbic network that are common sites of epilepsy foci and are implicated in both the pathophysiology of MDD and epilepsy (
6,
7). These regions include the anterior cingulate, the orbitofrontal cortex (OFC), the amygdala, and the hippocampus. We examined resting-state iEEG data across this network to identify neural features that differentiate patients with and without self-reported symptoms of depression.
Methods
A total of 13 adult patients undergoing surgical treatment for medication-refractory epilepsy who were implanted with intracranial grids, strips, or depth electrodes as part of their clinical evaluation for epilepsy surgery were included in the study. We used the Patient Health Questionnaire-9 (PHQ-9) (
10), a 9-item self-report instrument, to screen participants for a high burden of depressive symptoms (score ≥10). The first 24 hours of resting-state neural recordings were used to maximize signal quality. This study was approved by the institutional review board of the University of California at San Francisco, and written informed consent was obtained from all study subjects.
The Natus EEG clinical recording system (Natus Medical, Pleasanton, Calif.) was used to collect iEEG data at a 1–2 kHz sampling rate. Offline analysis was conducted with custom scripts in MATLAB (MathWorks, Natick, Mass.) and Python (Python Software Foundation, Wilmington, Del.). Standard iEEG preprocessing techniques were used, including application of a 2- to 250-Hz bandpass filter, notch filters at line noise frequency (60 Hz) and harmonics, down sampling to 512 Hz, and common average referencing. All data were manually cleaned of artifacts, seizures, epileptiform activity, and sleep in 30-second intervals under the supervision of one study author (AK), who is board certified in clinical neurophysiology and sleep medicine and was blind to high-depressive symptoms categorization. Only electrode contacts that were verified by MRI as being correctly positioned in the region of interest were used for analysis. Continuous waveform transformation with the Morlet wavelet transform method (
11) was performed in 30-second intervals to obtain power spectra in five frequency bands (delta=2–4 Hz, theta=4–7 Hz, alpha=8–12 Hz, beta=13–30 Hz, and gamma=31–70 Hz). Relative power was calculated by dividing the power of each frequency band by the total power for each electrode.
Principal component analysis was performed on the z-scored mean relative power across time and electrodes within a region and combined across study subjects (
4). The principal component analysis is used to address collinearity among EEG measures. It solves for linear combinations of the predictor variables, which are uncorrelated and then used as the predictor variables instead of the true variables. Stepwise linear and logistic regression models were performed with the PHQ-9 score or the presence of high depressive symptoms, respectively, as the outcome variable and principle components of the frequency spectral measures derived from the iEEG as the independent variables. Components were entered stepwise into the regression analysis. Because the principal component analysis is a linear transformation of the original dimensions, power units were maintained before and after it was applied. The composite score represents a linear combination of the original power estimates.
To determine the ability of the biomarker to correctly classify patients with and without high depressive symptoms, we carried out preliminary analyses of accuracy, sensitivity, and specificity by using the standard leave-one-out cross-validation method. This involved developing a model in a subset of all patients except one, and testing the model in the remaining study subject, performing multiple rounds of cross-validation until all participants were left out and then averaging the validation results over the rounds to estimate a final predictive model (
4).
Results
The demographic and clinical characteristics of the study sample are summarized in
Table 1. Among the 13 participants, 62% (N=8) had high depressive symptoms. The high-depressive symptoms group and the control group with minimal depressive symptoms were similar with regard to age (mean age=33.0 years [SD=5.93] and 33.4 years [SD=11.06], respectively). Eighty-eight percent of patients in the high-depressive symptoms group had temporal lobe epilepsy compared with 60% in the control group. Female participants comprised 50% (N=4/8) of the high-depressive symptoms group and 80% of the control group (N=4/5). In the high-depressive symptoms group, 88% of patients went on to receive surgical intervention compared with 80% in the control group) (chi-square or Kruskal-Wallis test, p>0.05). No participants were taking psychiatric medications. All patients were taking antiepileptic medications that were being tapered. There was no significant difference in the type of antiepileptic medications across the two study groups (chi-square or Kruskal-Wallis test, p>0.05).
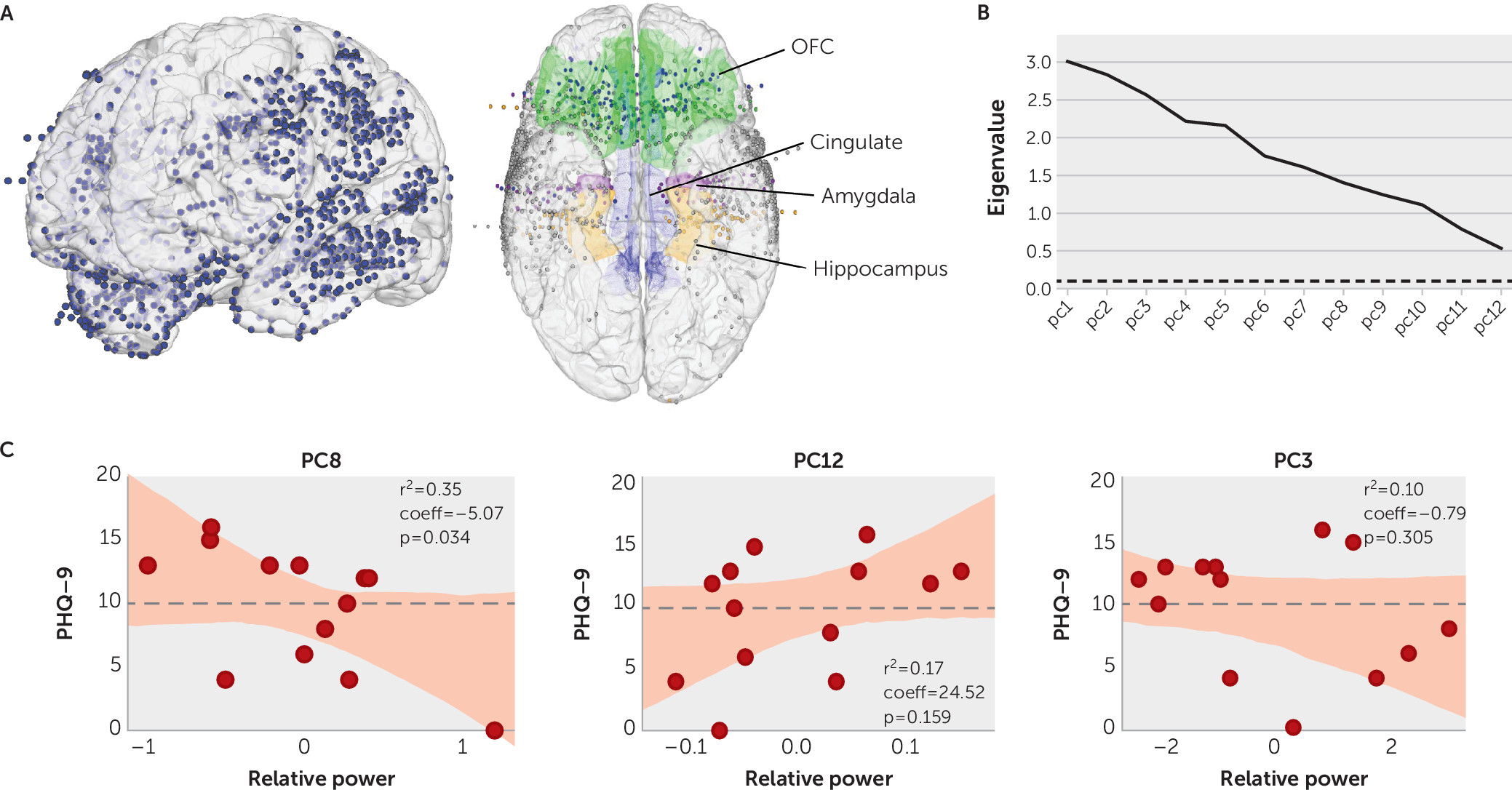
The recording locations across participants is shown in
Figure 1A. Principal component analysis yielded 12 principal components with eigenvalues >0.1 (
Figure 1B). Component loadings across the 12 principal components are presented in Table S1 in the
online supplement. A forward-stepwise linear regression model was a statistically significant predictor of the PHQ-9 score, with three principal components accounting for 62% of the variance (p=0.03, R
2=0.62, and leave-one-out cross-validation: pseudo R
2=0.29). We found that principal component 8 alone accounted for the majority (35%) of this variance (R
2=0.35, p=0.034, coeff=−5.07) (
Figure 1C). The other two principal components in the model contributed about equally (principal component 12: 17%; principal component 3: 10%).
We then carried out a dichotomous analysis to assess whether a set of neural features, represented by the principal components, could classify patients with high depressive symptoms from patients without high depressive symptoms. A forward-stepwise logistic regression model was a statistically significant predictor of high-depressive symptom status, in which principal component 3 was a significant contributor (R
2=0.35, p=0.014), with an odds ratio of 2.7. A leave-one-out cross-validation method, again, revealed acceptable generalization (pseudo R
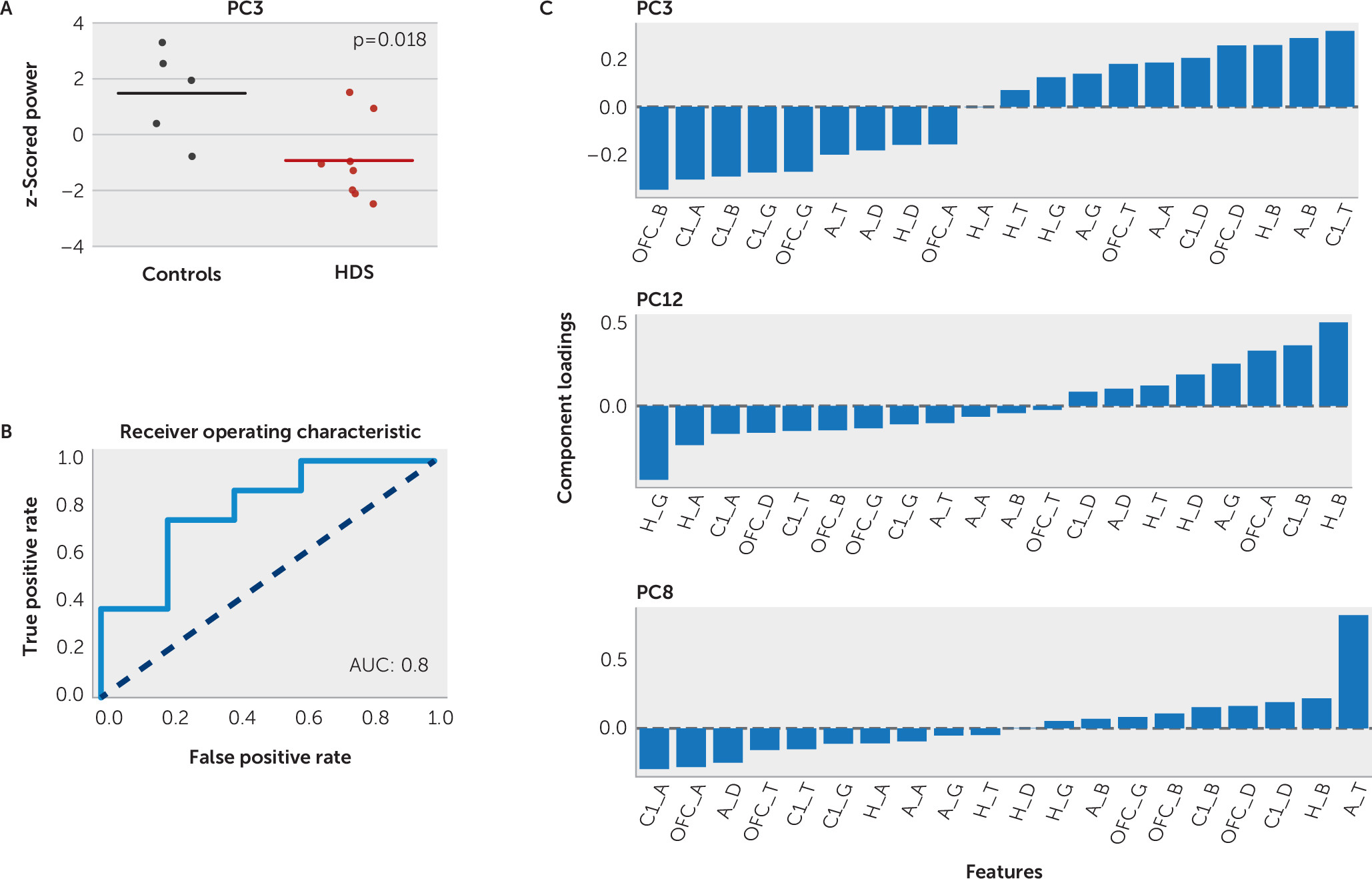
2=0.27). Principal component 3 was significantly lower in the high-depressive symptoms group compared with the control group (–0.93 and 1.48, respectively; two-sample t test, p=0.02). The mean power within principal component 3 and the range across individual study subjects for each group is shown in
Figure 2A. A balanced logistic classifier model with principal component 3 alone correctly classified 78% of patients as belonging to the high-depressive symptoms group or the control group (sensitivity, 75%; specificity, 80%; area under the curve [AUC]=0.8, leave-one-out cross validation) (
Figure 2B). These results provide initial evidence that principal component 3 was a significant modest, but reliable, factor in identifying most study subjects with depressive symptoms. There were some patients who did not follow this pattern and whose symptoms could represent a depression subtype that would be of interest to explore in a larger sample. The mean power within principal components 8 and 12 was not significantly different across the two study groups (p=0.24 and 0.28, respectively, two-sample t test).
Principal component 3 was common to both the logistic and linear regression models. It was heavily dependent on relative beta power across the entire corticolimbic network. Component loadings of principal component 3 indicated enhanced OFC and cingulate beta power in high depressive symptoms and decreased hippocampal and amygdala beta power (
Figure 2C). A balanced logistic classifier model with these four beta features alone correctly classified 64% of patients (sensitivity, 67%; specificity, 70%; AUC=0.7, leave-one-out cross validation), suggesting that corticolimbic beta power contributed most strongly to the classification dependent on principal component 3. In addition, high component loadings were observed for cingulate alpha (positively correlated with PHQ-9 scores) and theta power (negatively correlated with PHQ-9 scores), replicating previous findings that suggest the importance of cingulate activity in depression. Principal components 8 and 12 were in line with principal component 3 and also dependent on beta and theta power as well as cingulate activity (
Figure 2C).
Discussion
By using direct neural recordings, we identified a putative biomarker of self-reported depressive symptoms in medication-refractory epilepsy that was correlated with depression symptom severity and could correctly classify the majority of patients with and without high depressive symptoms (AUC=0.8). To our knowledge, this is the first study of a biomarker of psychiatric symptoms comorbid with focal epilepsy. Larger studies are required to validate these preliminary findings, assess their diagnostic utility in MDD, and evaluate their effectiveness for treatment stratification.
While a single brain circuit unique to depression in epilepsy is unlikely, previous studies support a degree of commonality across circuitry that underlies depressive symptoms (
8,
12,
13). Our results suggest that it may be possible to identify and extract this commonality, even with a small sample of patients with intracranial recordings. Our classification results are consistent with many larger functional MRI brain-based biomarkers (
14–
17). The classification was heavily dependent on power in the beta frequency band (13–30 Hz) throughout the corticolimbic network. Relative beta power was enhanced in the OFC and cingulate and decreased in the amygdala and hippocampus. Beta power is generally considered a marker of activation or arousal (
18) and has been identified as a marker of depression in previous studies, although the direction of the effect has been mixed (
19–
21). Fingelkurts et al. (
22) found that beta brain oscillations throughout the posterior cortical region characterized patients with depression, which was hypothesized by the authors to be a result of greater anxiety measured in these patients compared with control subjects. Similarly, a recent iEEG study of patients with epilepsy found that increased amygdala-hippocampal coherence variance in the beta band was correlated with worsening mood in a majority of study subjects (
13).
In addition to specific differences in beta power, we found enhanced cingulate alpha power and reduced cingulate theta power to be important contributors in distinguishing high depressive symptoms. Theta and alpha power within the limbic regions were also prominent contributors to the variance across PHQ-9 scores. Previous findings suggest that alterations in the cingulate in these frequency bands reflect disrupted limbic pathways (
23), may serve as markers of MDD (
24), and are modulated by antidepressant treatment (
25,
26). Indeed, alterations in theta and alpha bands have been most consistently reported in quantitative EEG studies of MDD (
25,
27–
35). These include early studies that reported alpha asymmetry reflected by increased left frontal alpha power (
33,
34), although this finding was subsequently reported to lack temporal stability (
35). In addition, subsequent work identified combined measures of alpha and theta power (antidepressant treatment response index [
27,
28] and a measure of theta power, i.e., theta cordance [
29]) as promising biomarkers of treatment response (
25,
30,
31,
36,
37). Other studies have investigated network organization in MDD by examining spectral coherence to estimate network connectivity (
38). One such study identified an overall stronger connectivity across brain regions in theta and alpha bands in patients with depression compared with nondepressed control subjects (
38). A subsequent study extended these findings and reported higher theta and alpha coherence in patients with MDD over healthy control subjects, especially within long-range connections between frontal regions and temporal and parietooccipital regions, and locally higher beta coherence within frontal and temporal regions (
24). Although the scope of the present study was limited to power features over coherence, we may speculate that our findings reflect both disrupted local (beta) and long-distance (alpha and theta) organization across the limbic network. In line with this hypothesis, network analysis with graph theoretical approaches has shown both inter- and intranetwork connectivity disruptions in emotion, attention, and cognitive networks in patients with depression (
39,
40).
While previous studies have relied on source localization of scalp EEG data to draw conclusions about spectral power within deeper structures, in the present study we employed intracranial recording and can, therefore, provide more definitive spatial localization of activity. As a result, this study extends current findings to link neural features from deep and cortical structures demonstrating a consistent and reciprocal relationship of spectral activity across the limbic network and supporting a focus on the cingulate in MDD.
An inherent limitation of working with iEEG recordings is the inconsistent placement of electrodes, which leads to challenges in grouping study subjects that have common recordings. Our sample size was small, because our goal was to identify a circuit-level biomarker, and patients were therefore included only if they had iEEG electrodes in the same four brain regions. As a result of the limited sample size, it was not feasible to train and test the regression models on independent data sets, and thus we used leave-one-out cross validation to maximize the amount of data available to learn discriminating features. Another limitation was the use of stepwise regressions, which were used to automatically select discriminatory variables from our larger set of principal components but have the potential for overfitting. Cross validation was performed in conjunction with the regressions to test the performance stability of the model and demonstrated consistent, but more moderate, correlations. Finally, it also remains possible that differences in epileptiform activity or the type of epilepsy contributed to the observed power changes, even after we removed epileptiform activity from the neural data.
While these results are preliminary, a common-circuit model that characterizes the majority of patients advances our understanding of depression and could serve as the substrate for larger studies that are aimed to replicate these findings directly in MDD and investigate the development of novel, personalized treatment strategies that specifically target dysfunctional networks.