Tourette’s syndrome affects slightly less than 1% of school-age children and 0.5% of adults (
1). The tics associated with Tourette’s syndrome can have significant effects on the academic and social development of children, as well as affecting their overall self-esteem and mental health. Although most children experience a decrease in their tics during adolescence, the worst symptoms usually are experienced by adults with intractable Tourette’s syndrome (
2,
3).
There are significant limitations to the available treatments for Tourette’s syndrome in terms of both efficacy and side effects. Currently, three antipsychotic agents are the only medications approved by the U.S. Food and Drug Administration for Tourette’s syndrome: aripiprazole, haloperidol, and pimozide. Second-generation antipsychotic agents are also widely used in the treatment of Tourette’s, even though only risperidone has demonstrated efficacy in multiple placebo-controlled clinical trials (
4–
6). Antipsychotic agents, although the most effective agent in reducing tics, are not used as a first-line treatment for tics (especially in children) in the United States because of the significant side effects associated with their use (
4–
6). Alpha-2 agonists, such as guanfacine and clonidine, are often used as a first-line pharmacological treatment for tics in the United States but have limited evidence for actually improving tic symptoms (
7,
8). Behavioral therapy, such as comprehensive behavioral intervention for tics, has strong evidence of efficacy for both children and adults with Tourette’s syndrome (
9,
10). However, the efficacy of comprehensive behavioral intervention for tics remains unclear when practiced outside experienced academic centers specializing in the treatment of the disorder, and availability remains a major challenge. New treatments for Tourette’s syndrome are urgently needed, because the disorder represents an unmet medical need.
Several lines of evidence suggest that cannabis (Cannabis
sativa) and Δ
9-tetrahydracannabinol (Δ
9-THC) may be effective in the treatment of tic disorders. Anecdotal case reports have long suggested that smoking marijuana may improve tic symptoms (
11–
13). Standardized interviews of 17 consecutive patients with Tourette’s syndrome seeking treatment in Germany with previous cannabis exposure reported that 82% experienced a reduction or complete remission of tic symptoms when smoking cannabis (
14). Δ
9-THC, the principal active constituent of cannabis, has been studied in randomized, placebo-controlled trials in Tourette’s syndrome (
14). A double-blind, placebo-controlled crossover trial of 5–10 mg of Δ
9-THC in 12 adults with Tourette’s syndrome demonstrated a significant reduction in measures of tic severity and global improvement with Δ
9-THC compared with placebo. Tourette Syndrome Symptom List (TSSL) ratings of tic severity were significantly reduced when participants were given Δ
9-THC (mean=12.5 points [SD=11.0]) compared with placebo (mean=2.5 points [SD=7.0]; p=0.015) (
15). A 6-week, double-blind, placebo-controlled trial of 24 adults with Tourette’s syndrome also demonstrated a significant benefit of Δ
9-THC compared with placebo. Participants randomly assigned to Δ
9-THC experienced significant reduction of their tic symptoms compared with placebo after the first 2 weeks of treatments that continued throughout the 6-week trial. These improvements vanished when participants discontinued Δ
9-THC (
16). The treatment effect size observed for Δ
9-THC was considerably larger than that observed in placebo-controlled trials of currently available treatments for Tourette’s syndrome.
Cannabinoid receptor type 1 (CB
1) receptors may be involved in the pathophysiology of Tourette’s syndrome. Biological evidence also suggests that the brain’s cannabinoid system may contribute to the pathophysiology of Tourette’s and other movement disorders (
17,
18). CB
1 receptors and endocannabinoids are highly expressed in the basal ganglia and appear to have an important role in modulating dopaminergic motor circuits (
18). Specifically, CB
1 receptors are expressed presynaptically on medium-spiny neurons–striatal neurons projecting in both the direct (substantia nigra pars reticulata and the globus pallidus pars interna) and indirect (globus pallidus pars externa) pathways (
19). Activation of CB
1 receptors in combination with D
1 receptors of the direct pathway serves to decrease adenyl cyclase release within projection neurons. By contrast, activation of CB
1 receptors in combination with D
2 receptors serves to stimulate adenyl cyclase in projection neurons of the indirect pathway (
18). Although CB
1 receptors are not expressed by most striatal GABAergic interneurons, CB
1 receptors are expressed by parvalbumin positive interneurons and some cholinergic interneurons (
19). Parvalbumin positive and cholinergic striatal interneurons have been demonstrated to be significantly reduced in postmortem studies of individuals with Tourette’s (
20).
The major limitations of both cannabis and dronabinol (a synthetic form of Δ
9-THC) use are the adverse psychoactive side effects they induce in higher doses. The psychoactive effects of Δ
9-THC are primarily mediated by its activation of CB
1 G-protein-coupled receptors, which result in a decrease in the concentration of the second messenger molecule cAMP through inhibition of adenylate cyclase (
21). These adverse events include but are not limited to dizziness, somnolence, paranoia, cognitive impairment, nausea, and vomiting. These side effects represent a major constraint to the successful implementation of Δ
9-THC as a therapeutic agent in Tourette’s syndrome. Thus, in order to harness the therapeutic potential of THC for patients with Tourette’s, there is a need to reduce the accompanying adverse effects.
We conducted this initial open-label trial to examine the safety and tolerability of THX-110 (a combination of Δ
9-THC and palmitoylethanolamide [PEA]), as well as its effects in tic symptoms in adults with Tourette’s syndrome. By using THX-110, we hoped to use the entourage effect to deliver the therapeutic benefits of Δ
9-THC in reducing tics with decreased psychoactive effects by combining with PEA. The basic idea of the entourage effect is the endocannabinoid regulation by which multiple endogenous cannabinoid chemical species display a cooperative effect in eliciting a cellular response. PEA is a lipid messenger known to mimic several endocannabinoid-driven activities, although it does not bind the classical CB receptors. On the basis of an activity enhancement of other physiological compounds, by potentiating their affinity for a receptor or by inhibiting their metabolic degradation, PEA may indirectly stimulate the effects of both phytocannabinoids and endocannabinoids, either by its role as an agonist of the transient receptor potential vanilloid type 1 (TRPV1), peroxisome proliferator-activated receptor-alpha, or the cannabinoid receptors (
6). PEA’s capacity to exert entourage effects is derived from its ability to affect multiple targets within the body, improve the absorption of active ingredients, and minimize adverse side effects (
7). The entourage effect may also be accounted for by the pharmacological actions of PEA. On the basis of an activity enhancement of other physiological compounds, by potentiating their affinity for a receptor or by inhibiting their metabolic degradation, PEA may indirectly stimulate the cannabinoid receptors and by doing so may increase the absorption of cannabinoid compounds, such as THC (
22,
23). This is supported by the effect of PEA on the endocannabinoid anandamide (arachidonoylethanolamide [AEA]), which mimics THC agonist effect on CB
1/CB
1 receptors. PEA was found to enhance the hypotensive responses to AEA. Subeffective doses of AEA induce decreased blood pressure only when coadministered with PEA; AEA also induces vasorelaxation only in the presence of PEA. The latter was a result of PEA agonist activity on TRPV1 receptors (
24). As PEA potentiates the effect of AEA, it is plausible to assume that it may exert the same effect on THC. Thus, the coadministration of dronabinol with PEA as presented in the proposed investigational product is suggested to act under an entourage effect, resulting in a safer and more effective therapy than the use of dronabinol alone. As THC was found to ameliorate Tourette’s syndrome symptoms, the coadministration of PEA with dronabinol (THC) is expected to improve the safety and beneficial effect of dronabinol in Tourette’s syndrome.
METHODS
Overview
We conducted a 12-week, uncontrolled trial of THX-110 in 16 adults with Tourette’s syndrome. Participants received THX-110 for the duration of the trial at a maximal daily dose of 10 mg of Δ9-THC and a constant daily dose of 800 mg of PEA. Participants could elect to continue to receive continued THX-110 treatment for 24 additional weeks while undergoing monthly clinical and safety assessments as well as at the conclusion of the 12-week trial. Our goals for this pilot study were to provide initial safety, feasibility, and tolerability data on THX-110 in a Tourette’s syndrome population and provide data on THX-110 effects on tic severity in order to make a more informed decision regarding the appropriate sample size and design of a larger, double-blind, placebo-controlled clinical trial to examine efficacy. The primary outcomes from this trial were the Yale Global Tic Severity Scale (YGTSS) total tic score to examine Tourette’s syndrome severity, the proportion of study subjects who elected to continue on to the extension phase of the trial (as a measure of patient-perceived benefits versus side effects of THX-110), and the number of patients who dropped out as a result of adverse effects and lack of efficacy.
Participants
Sixteen adults were recruited through the Tourette’s Syndrome/Obssessive-Compulsive Disorder (OCD) Clinic at the Yale Child Study Center. The Tourette’s Syndrome/OCD Clinic is a tertiary referral clinic for Tourette’s syndrome at a large academic center. Additional participants were recruited through direct emails from individuals reading the clinicaltrials.gov website and referrals from known Tourette’s syndrome providers in the New York, New Jersey, Connecticut, Massachusetts, and Rhode Island areas through provider letters informing them of the ongoing study. Study subjects were compensated $500 for their participation in the 12-week study and compensated $50 for each follow-up assessment.
To meet inclusion criteria for the trial, individuals had to be 18–60 years old, meet DSM-5 criteria for diagnosis of Tourette’s syndrome, have significant current tic symptoms (YGTSS total tic score ≥22 at baseline) (
25), and be on a stable psychiatric medication regimen for a minimum of 4 weeks prior to beginning the trial. In addition, female participants were required to use an accepted method of birth control at the start of the trial and throughout the trial. Exclusion criteria for the trial were comorbid bipolar disorder, psychotic disorder, substance use disorder, developmental disorder, or intellectual disability (IQ <70); recent change (<4 weeks) in other medications that have potential effects on tic severity (e.g., alpha-2 agonists [guanfacine, clonidine, or prazosin], selective serotonin reuptake inhibitors, clomipramine, naltrexone, lithium, anxiolytics, topiramate, or baclofen; medication change is defined to include dose changes or medication discontinuation); recent change in behavioral treatment for Tourette’s syndrome or comorbid conditions (i.e., OCD) within the past 4 weeks or initiation of behavioral therapy for tics within the past 12 weeks; positive pregnancy test or drug screening test (including cannabis); history of cannabis dependence; and significant medical comorbidity. Participants were required to test negative for cannabis on a urine drug screen to be eligible for this study.
Interventions
Participants were assigned to receive once-daily THX-110 (maximum daily dose, 10 mg of Δ9-THC and 800 mg of PEA) for 12 weeks. Participants were titrated up on Δ9-THC dose during the first week of the trial (2.5 mg Δ9-THC for 3 days and then 5 mg Δ9-THC for 4 days increasing to 10 mg Δ9-THC for the remainder of the trial). PEA dose remained constant throughout the trial. Δ9-THC was increased to 10 mg at the week 1 assessment if the study subject was tolerating the 5 mg dose of Δ9-THC, and the Δ9-THC was reduced based on patient side effects. Δ9-THC dose could be titrated based on investigator discretion when evaluating side effects of medications, benefits on tic severity, and subject preference. THX-110 was initially given in the morning (as previous studies suggested, Δ9-THC may have an immediate effect on tics) but was often switched to evening dosing or split into twice-daily dosing in order to improve tolerability.
Assessments
After an initial phone screen to rule out obvious exclusions from the study protocol, potential study subjects had an initial evaluation that was performed by a multidisciplinary clinical team. In addition to a standard clinical evaluation consisting of history and mental status examination, participants received a clinical diagnostic interview using the Structured Clinical Interview of DSM-IV. Participants underwent detailed ratings of tic severity and common comorbid conditions as conducted by an experienced rater (M.D.-level training). Ratings were conducted at baseline and every 2 weeks throughout the initial 12-week trial. Clinical ratings included measures of tic severity (YGTSS total tic score) (
25), Premonitory Urge for Tics Scale (PUTS) and Tourette Syndrome Symptom List (TSSL) (
26), OCD severity (Yale-Brown Obsessive-Compulsive Scale [Y-BOCS]) (
27,
28), attention deficit hyperactivity disorder (ADHD) severity (Conners’ Adult Attention Deficit Hyperactivity Rating Scale) (
29); depression severity (Hamilton Rating Scale for Depression [HAM-D]) (
30), anxiety severity (Hamilton Rating Scale for Anxiety [HAM-A]) (
31), and overall improvement (Clinical Global Improvement Scale) (
32). Adverse effects of medication were assessed systematically when participants asked specifically about possible side effects at each visit (
33). Ratings of tic severity and adverse events was additionally evaluated 1 week after starting the study medication in order to determine dose adjustment of the study medication. A medical safety assessment, which included vital sign measurements, electrocardiogram, physical examination, and routine blood tests indicating physical health and urine drug screen and pregnancy test, was completed prior to study enrollment and every 2 weeks throughout the trial. Blood tests consisted of electrolytes, liver function tests, and complete blood count. Urine drug screens for cannabis were also used to track compliance with the study medication; if study participants tested negative for cannabis in urine drug testing, it was assumed they were not regularly taking the study medication and were removed from the trial. At the conclusion of the 12-week trial, participants were given the option of continuing on the study medication for an additional 6 months during which they received addition efficacy and safety ratings every 4 weeks and were compensated $50 for each study visit and continued to be prescribed the study medication.
Data Analysis
We set a priori criteria for the go/no-go decision for further investigation of this treatment based on the results of this trial, which is not a true efficacy trial but rather a phase II pilot study. Trial results provide sufficient encouragement to move forward to a phase III trial. These a priori criteria included measures of feasibility: average treatment compliance with the Δ9-THC/PEA combination of at least 80% (via positive urine drug screen) during the trial; tolerability and safety: attrition due to adverse effects of four or fewer study subjects receiving treatment; exacerbation of tics: four or fewer participants had a greater than 20% worsening of tic symptoms while in the trial; and efficacy: an average improvement of YGTSS severity of greater than 10% during the course of the 12-week trial. Assuming all go/no-go decision points were met in the trial, we would additionally use the initial pilot data to design the most appropriate phase III trial design, in terms of dosing strategy and duration of treatment. The last outcome of interest was the proportion of participants who would elect to continue receiving the medication in the 6-month extension phase of the trial.
Our primary outcome for efficacy was the YGTSS total tic score. Specifically, we examined difference in tic severity as assessed by YGTSS (across all study subjects and specifically those assigned to active medication) between week 0 (baseline) and week 12 (endpoint) to determine the time at which treatment benefits likely plateau. Our primary analysis involved a generalized linear model with YGTSS total tic score as the dependent measure and time as a repeated measure within subject. We were interested in whether there was a significant benefit over time with treatment. We were additionally interested in the percentage improvement in tic symptoms and effect size (improvement from baseline) at each time point throughout the trial (in order to determine the optimal trial length for a definitive phase III study). TSSL and PUTS ratings were analyzed in a similar manner. We also conducted paired t tests based on beginning and endpoint assessments to examine all measures of tic severity and secondary measures of OCD, ADHD, depression, and anxiety. Rates of common (experienced by at least two participants) and significant side effects are reported, as well as reasons for dropouts from the trial.
RESULTS
Participants
The characteristics of the 16 participants included in this trial are reported in
Table 1. This sample of adults (mean age=35.0 years [SD=13.0]; range, 18–56 years) had severe tic symptoms at baseline (YGTSS total tic score=38.1 [SD=8.6]; range, 20–50); worst-ever total tic score=45.4 [SD=5.5], range, 36–50). The mean age at onset for these tic symptoms was 8.4 years (SD=4.2), and participants had a mean duration of illness of 26.6 years (SD=13.5; range, 6–50 years). These tics had persisted despite previous evidence-based treatments for TS, including antipsychotics (N=16, 100%), alpha-2 agonists (N=14, 88%), VMAT2 inhibitors (N=5, 31%), benzodiazepines (N=12, 75%), and topiramate (N=8, 50%). Three of 16 participants were not taking medications for psychiatric conditions (19%). Ten participants (63%) were taking other medications to treat tic disorders, including antipsychotic medications (N=7, 44%), alpha-2 agonists (N=4, 25%), vesicular monoamine transporter inhibitors (N=1, 6%), and benzodiazepines (N=5, 31%). Twelve participants (75%) had comorbid OCD, three had comorbid ADHD (19%), five had a comorbid anxiety disorder (31%), and six had a history of major depressive disorder (38%).
Table 1 reports the baseline characteristics for each participants included in our trial.
Tic Symptoms
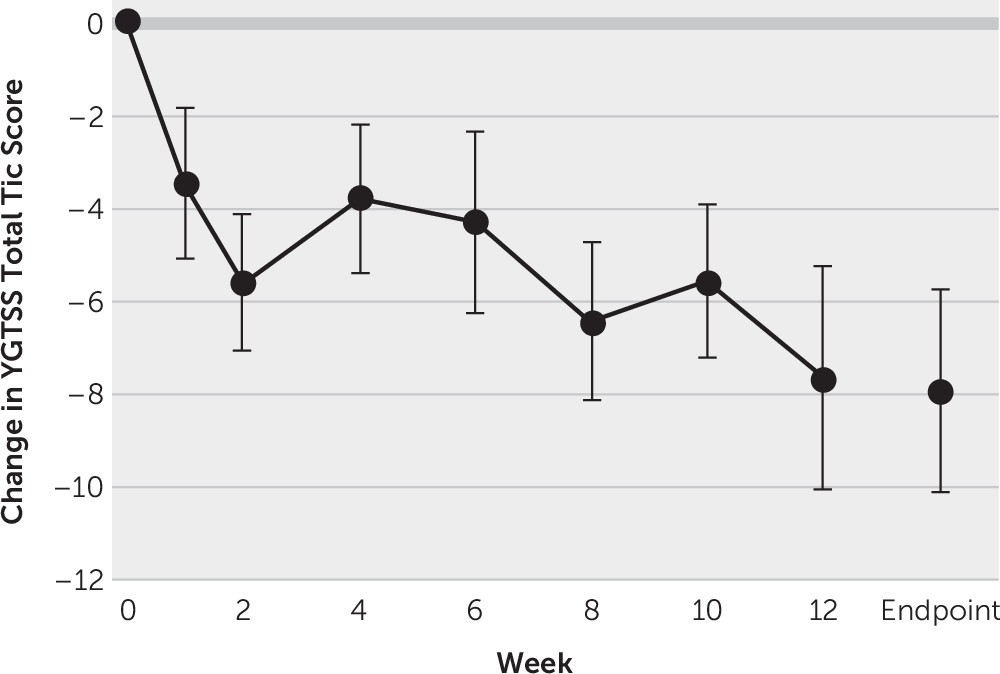
Figure
1 depicts the improvement of tic symptoms throughout the course of the trial when treated with THX-110 treatment. Tic symptoms significantly improved over time with THX-110 treatment (general linear model time factor: F=3.06, df=7, 91, p=0.006). Improvement in tic symptoms was statistically significant within 1 week of starting treatment (first assessment point: mean YGTSS improvement=3.5 [SD=6.5], 95% CI=0.1, 6.9, t=2.2, df=15, p=0.047), and the improvement remained significant and increased throughout the 12 weeks of the trial. The maximal improvement in tic symptoms was observed at 12 weeks of treatment (mean YGTSS improvement=7.6 [SD=9.5], 95% CI=2.5, 12.8, t=3.2, df=13, p=0.007). The mean YGTSS improvement at endpoint using last observation carried forward for all participants was 7.9 ([SD=8.6], 95% CI=3.3, 12.5, t=3.2, df=15, p=0.002). Four participants (25%) experienced a treatment response as defined by a greater than 35% improvement in tic symptoms during the trial, whereas six participants (38%) experienced at least a 25% improvement in tic symptoms. The average improvement in tic symptoms was 20.6% ([SD=24.3%], 95% CI=7.7%, 33.6%, t=3.4, df=15, p=0.004) during the course of the trial.
There was also a significant improvement between baseline and endpoint on other measures of tic symptoms (mean TSSL improvement=5.9 [SD=9.8], 95% CI=0.7, 11.2, t=2.4, df=15, p=0.03) but not premonitory urges (mean PUTS improvement=2.3 [SD=7.1], 95% CI=−1.4, 6.1, t=1.3, df=15, p=0.21).
Comorbid Symptoms
THX-110 treatment did not demonstrate significant effects on any of the comorbid symptoms in participants with Tourette’s syndrome. ADHD (mean Conners’ ADHD Rating Scale improvement=1.9 [SD=13.3], 95% CI=−5.2, 9.0, t=0.6, df=15, p=0.58), OCD (mean Y-BOCS improvement=2.7 [SD=7.9], 95% CI=−1.5–6.9, t=1.4, df=15, p=0.19), depression (mean HAM-D improvement=2.9 [SD=7.7], 95% CI=−1.2–7.0, t=1.5, df=15, p=0.15), and anxiety symptoms (mean HAM-A improvement=2.9 [SD=8.4], 95% CI: −1.6–7.3, t=1.4, df=15, p=0.19) all modestly improved during the course of the trial, but not to a statistically significant degree.
Table 2 depicts ratings of tic symptoms and comorbid conditions at baseline and endpoint of the trial.
Safety and Tolerability
Two participants (12.5%) discontinued the trial early. One participant discontinued because he felt the medication was not helping his tic symptoms (subject 10); another discontinued because of drowsiness and fatigue (subject 2) related to taking the study medication. Subject 11 also had a negative urine toxicology for THC and its metabolites at his last assessment visit, suggesting that he had not been taking the study medication during the previous several weeks. The average daily dose of the medication was 6.9 mg [SD=2.8] of Δ
9-THC, and all participants received 800 mg of PEA.
Table 3 depicts the side effects experienced by participants enrolled in the trial. Mild side effects that occurred typically for a couple hours after taking study medication were experienced by all participants. These side effects were experienced during the course of dose escalation and maintenance but generally were not severe enough to warrant medication discontinuation. These side effects were minimized by shifting the majority of dosing of the medication administration to evenings and at times lowering the dosage of study medication. Side effects of moderate or greater severity that led to changes in the dosing of study medication were less common and typically included fatigue/drowsiness, feeling high, dizziness, and headache.
Table 2 shows the maintenance dose and dosing schedule for participants included in this trial. No significant laboratory abnormalities were experienced by any participant during the course of the trial.
Extension Phase
Twelve participants (75%) who entered the trial (and 12 of 14 [86%] who completed the acute treatment phase) elected to continue THX-110 treatment during the open extension phase of the trial. Seven of the 12 participants enrolling in the extension phase completed the additional 24 weeks of treatment with THX-110. During the extension phase, one participant dropped out prematurely from the extension phase due to a worsening headache that did not resolve after discontinuation of the study medication (subject 7). Two participants discontinued the study medication because they could no longer maintain the study follow-up visit schedule (subjects 6 and 8); subject 11 started the extension phase but was dropped because he had a negative urine toxicology at week 12, indicating that he was likely not taking the study medication. One subject (
13) elected to drop out from the extension phase at week 28 to try alternative medications for tics. No participants experienced significant abnormalities or adverse health outcomes during the course of the extension phase.
DISCUSSION
In this study, participants demonstrated a significant improvement in tic symptoms over time with THX-110 (a combination of Δ9-THC and PEA) treatment. THX-110 treatment led to an average improvement in tic symptoms of roughly 20%, or a 7-point decrease in the YGTSS total tic score. This improvement translates to a large effect size (d=0.92) of improvement over time. Additionally, 25% of a refractory population of individuals with Tourette’s syndrome who had not responded to previous evidence-based treatments for tics experienced a significant treatment response in terms of tic symptoms. Seventy-five percent of participants who started the medication elected to continue in the open extension phase, and only two out of 16 participants (12.5%) dropped out of treatment early: one due to sedation and the other due to inefficacy (as a result of not taking the study medication). However, many participants experienced transient adverse effects of THX-110 in the trial that often required a dose adjustment. Although these initial data are promising, future randomized double-blind placebo-controlled trials are necessary to demonstrate efficacy of THX-110 treatment.
Many pharmacological agents that have demonstrated improvement in uncontrolled trials in Tourette’s syndrome have failed to demonstrate efficacy in placebo-controlled trials. Tic symptoms in Tourette’s syndrome have a fluctuating, waxing-and-waning course; patients with the disorder may enroll in trials and initiate treatment when their tics are bad or at their worst, and thus regression to the mean during uncontrolled trials such as this one cannot be ruled out. Nonetheless, this open trial demonstrated that THX-110 was well tolerated in adults with Tourette’s syndrome, especially when dosed at nighttime. Many participants believed that the THX-110 was helpful and elected to continue on the medication when given the opportunity, and all a priori go/no-go decision points for a larger double-blind placebo-controlled trial were met.
Our open trial of THX-110 treatment supports an emerging body of evidence suggesting that cannabinoid compounds may be effective for the treatment of Tourette’s syndrome. This research was initially spurred by the reports of many patients with Tourette’s suggesting that their tics improved when they smoked cannabis (
14). This improvement in symptoms is not reported with alcohol, opiates, cocaine, and other substances of abuse. Furthermore, two clinical trials have suggested the benefits of Δ
9-THC in a 12-subject single-dose, placebo-controlled crossover trial and a 24-subject 6-week parallel-group placebo-controlled trial (
15,
16). Additionally, multisite double-blind trials assessing the efficacy of cannabis-based compounds are needed to demonstrate efficacy and delivery mechanism of cannabis-based compounds, determine whether there are added benefits of compounds such as PEA that encourage the entourage effect, and determine whether other medications that can increase endocannabinoid level (e.g., monoacylglycerol-lipase or fatty acid amide hydrolase inhibitors) can also improve tic severity. A major challenge for these studies will be adequately blinding studies of THC compounds, which commonly produce side effects such as dry mouth, sedation, increased appetite, feeling high, dizziness, and so on that threaten to functionally unblind studies.
Although this trial showed a significant improvement of THX-110 treatment over time, it had several limitations. First, the trial was uncontrolled, and thus we do not have a comparison to account for the natural waxing-and-waning symptoms that often occur in Tourette’s syndrome. That being said, our sample was quite refractory to previously used evidence-based treatments, such as antipsychotic medications and alpha-2 agonists. Many of these participants had participated in previous trials of rTMS and other compounds that have failed to demonstrate improvement. Additionally, although participants also showed modest, nonsignificant improvement in several other secondary measures such as depression, anxiety, and OCD symptoms, we believe that this quite possibly was a secondary effect of the improvement in tics. Finally, given the small sample size of the trial, we have limited data on the safety of THX-110.
Nonetheless, this trial provided several important lessons that will inform a more definitive randomized double-blind placebo-controlled trial of the combination of THX-110. The benefits of THX-110 occurred fairly quickly after initiation of treatment (significant improvement within the first week), and for many participants the benefits of THX-110 were observed at fairly low doses of Δ9-THC, such as ≤5 mg. This observation raises the questions of whether use of PEA may allow for a reduced dose of Δ9-THC to be effective in the treatment of tics, and if combination Δ9-THC/PEA or Δ9-THC alone could be used not only as a chronic medication to help tics (as studied in this trial) but also as an acute short-term medication to be used during symptom exacerbation. Most participants reported transient mild to moderate adverse effects that were minimized when the proper dose was established (especially when given at night). Additionally, THX-110 was better tolerated when given primarily at night. We believe the results from this trial provide encouraging preliminary data to support the study of cannabis derivatives, and specifically the combination of Δ9-THC/PEA, in the treatment of Tourette’s syndrome. On the other hand, we believe it is premature to conclude demonstrated efficacy of THX-110 or even cannabis derivatives in general. Further multisite randomized placebo-controlled trials of cannabis derivatives are needed to demonstrate efficacy in Tourette’s syndrome. Additionally, the challenges raised by the difficulty in blinding trials raised by the psychoactive properties of many cannabis-derived compounds needs to be further appreciated in these trial designs. Incorporation of physiologic biomarkers and objective measures of symptoms (e.g., videotaped tic counts by blinded raters) may be particularly important when examining these medications with psychoactive properties that may be prone to reporting bias.