Functional neurologic disorder (FND) is a costly yet common reason for patient evaluation in outpatient neurology clinics (
1) and acute care settings for neurologic symptoms (
2,
3). The incidence of functional tics, a subset of FND, increased substantially during the coronavirus disease 2019 (COVID-19) pandemic. A 2021 article that aggregated observations from eight Tourette syndrome clinics around the world reported a 10- to 20-fold increase in new referrals for functional tics, particularly among adolescents, during the pandemic (
4).
Little is known about the expected clinical course and prognosticating factors of functional tics and FND triggered by global traumatic events. Outbreaks of FND have occurred following regional traumatic events, such as the Boston Marathon bombings (
5), and sociogenic situations, such as the case in which 19 students in a high school in Le Roy, N.Y., developed new-onset tics amid speculations about poor local water quality—many of these symptoms improved after the cessation of media attention (
6,
7). During the COVID-19 pandemic, onset of functional tics tracked exposure on social media, including TikTok and YouTube (
8–
12).
Among adults with FND, predictors of a good outcome include early treatment, early diagnosis, younger age, patient compliance with treatment regimen, and confidence in a positive treatment outcome (
13). Among pediatric patients, early treatment is also important (
14). Additionally, parental acceptance of the diagnosis affects recovery (
15), but patients’ impression of their own illness is likely to be an independent factor.
To further inform practitioners, patients, and families who encountered a diagnosis of functional tics during the COVID-19 pandemic, we performed a single-center, cross-sectional cohort study involving a review of electronic medical records (EMRs) and follow-up telephone surveys to determine degree of improvement at last follow-up among pediatric patients diagnosed as having functional tics during the COVID-19 pandemic. We explored factors that predict prognosis and evaluated differences in the perceptions of symptoms and recovery between patients and parents.
Methods
Patients
This study was approved by the University of Virginia Institutional Review Board (HSR210546). Initial data were collected from the EMR or a specialty clinic database. Inclusion criteria were a diagnosis of functional tics as determined by a board-certified pediatric neurologist and a diagnosis date between March 1, 2020, and December 21, 2021. A functional etiology was confirmed after review by a pediatric movement disorders specialist (J.G.). If the diagnosis was unclear, inclusion was determined following independent review by a second specialist (A.A.L.).
The primary outcome variables were patient- and guardian-reported Clinical Global Impression–Improvement (CGI-I) scores. These scores were aggregated with estimates of clinician-rated CGI-I scores from EMR documentation (A.M.) to allow for descriptive modeling of individual clinical courses and exploratory regression modeling. Secondary outcome variables included time from diagnosis to first improvement and presence of functional interference at most recent follow-up.
Additional demographic, historical, and phenomenological features were documented based on EMR review. After obtaining verbal informed consent and assent, guardians and patients were surveyed separately about agreement with the diagnosis, current symptoms, time to first improvement, and exposure to tics via social media or social contacts.
Data Analysis
CGI-I scores and time to first improvement are reported as medians with interquartile ranges. Ongoing interference from tics is reported as the percentage of patients affected. Individual clinical courses, including symptom onset, diagnosis, and improvement as represented by CGI-I scale scores over time, are graphically represented. The effect of diagnosis on improvement was evaluated with a Kaplan-Meier survival analysis.
To explore the effects of clinical factors on prognosis, univariate logistic regression models were used to assess the probability of significant improvement (CGI-I score <3) at the most recent interview or clinical encounter and any improvement (CGI-I score <4) with patients dichotomized according to time until initial improvement after diagnosis (≤1 month or >1 month). Variables of interest included age, exposure to social media depictions of tics, time spent on social media, report of stressors, presence of additional FND symptoms, comorbid anxiety or depression, time to diagnosis, medications prescribed for tics, acute care presentation, interference from tics at diagnosis, agreement with diagnosis, differences in parent-child responses (outcome variables assessed in the survey), ongoing exposure to tics through friends or social media, and whether the patient received comprehensive behavioral intervention for tics. Odds ratios with 95% confidence intervals and p values were computed for all models.
Agreement between patients and guardians regarding the diagnosis and agreement on CGI-I scores were assessed with McNemar and Wilcoxon signed-rank tests, respectively. Summary statistics, statistical tests, regression analyses, and visualizations were performed with GraphPad Prism, version 8.1.1, or R, version 4.0.0.
Results
Initial screening discovered 90 patients diagnosed as having FND; 29 of these patients had functional tics diagnosed by a board-certified pediatric neurologist and therefore met the inclusion criteria. All patients had at least one follow-up clinical encounter. Twenty dyads completed telephone interviews and were included in comparative analyses of the impressions of parents or guardians; at least one member of five dyads could not be contacted despite multiple attempts, at least one member of three dyads declined to complete the interview, and one dyad withdrew from the study after the guardian survey but before the patient survey. Descriptive statistics are provided in Table S1 in the online supplement. A majority of the patients were teenage girls (mean±SD age=15.8±1.4 years) with preexisting anxiety or mood disorders who reported life stressors and had been exposed to tics through social media (55%) or personal contacts (31%). Reported races and ethnicities were White (86%), Black (7%), Hispanic (3%), and “other” (3%). The most common phenomenological features included trunk or limb involvement, complex movements, a varied repertoire, context-dependent movements, and tics occurring during the examination (see Figure S1 in the online supplement). Features typical of Tourette syndrome, such as worsening of tics when alone, were rare. Prior to diagnosis, many patients had medications prescribed to treat presumed primary tic disorder and had already been receiving psychotherapy (see Table S1 in the online supplement). New treatments prescribed after diagnosis included cognitive-behavioral therapy and comprehensive behavioral intervention for tics.
The primary outcome measures of parent- and guardian-rated CGI-I scores are provided in
Table 1 with other outcome measures. At the final follow-up, 21 of 29 patients (82%) reported at least some improvement since diagnosis. Three of the patients who reported no improvement since the diagnosis had improved before the diagnosis. Seventeen of 29 patients (59%) were much or very much improved. Despite these improvements, ongoing interference was common. Examples of reported interference included having tics interrupt sentences and engaging in fewer social activities because of tics.
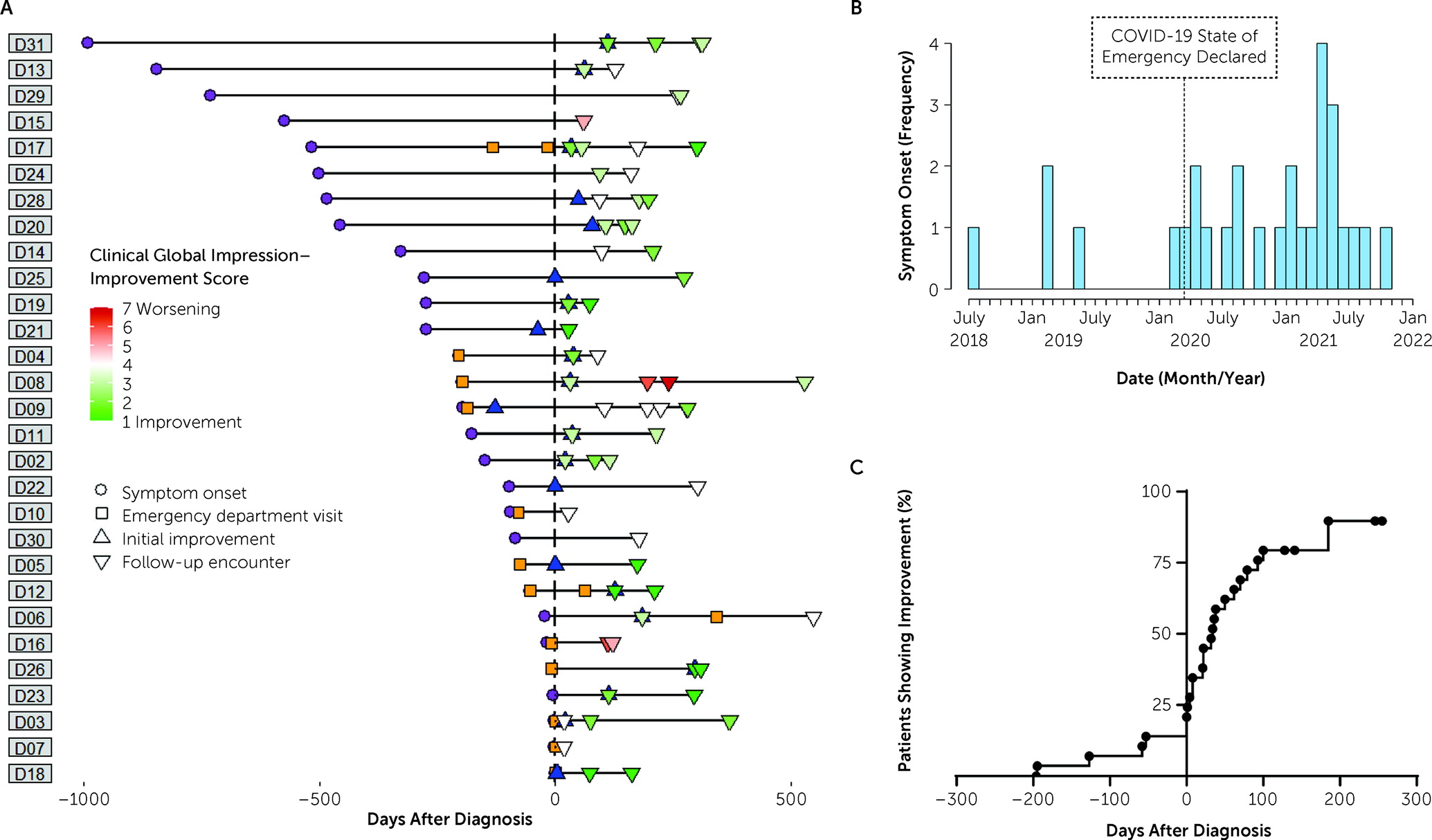
The clinical course of each patient, including symptom onset, acute care visits, diagnosis, initial improvement, and follow-ups, is shown in
Figure 1A. The median date of symptom onset was January 1, 2021 (interquartile range [IQR] from July 30, 2020, to July 26, 2021), nearly 8 months after COVID-19 was declared a national emergency in the United States (
Figure 1B). Nearly half (48.3%) of the initial presentations among our sample were to emergency departments or urgent care clinics. The median date of diagnosis was June 7, 2021 (IQR from May 5, 2021, to September 21, 2021), with median time from symptom onset to diagnosis of 197 days (IQR=54–456). Although some patients improved before receiving a diagnosis, a majority of patients improved rapidly after receiving a diagnosis, with a median time until initial improvement of 21 days (IQR=0–61) after diagnosis (
Figure 1C).
Univariate regression models assessing factors predicting improvement within 1 month following diagnosis identified age at time of diagnosis as a positive prognostic factor, with each additional year of age increasing the probability of improving by a factor of more than 2 (odds ratio=2.11/year, 95% CI=1.03–4.32, p=0.041). An inverse association between time from symptom onset to diagnosis and improvement within 1 month of diagnosis was suggested with each month delay decreasing odds of recovery (odds ratio=0.89/month, 95% CI=0.78–1.00, p=0.055). No factors predicted final CGI-I scores, although age at diagnosis and patient agreement with diagnosis were borderline-positive correlates. The full results of regression analyses are available in Table S2 in the online supplement.
Discussion
This single-center, cross-sectional cohort study demonstrated rapid improvement but not resolution of functional tics after diagnosis for a majority of pediatric patients, although many patients continued to note long-term impairment from tics.
Our findings corroborate recent reports based on clinician-rated tic severity scales by Howlett et al. (
16). In addition to clinician ratings, we evaluated patient and parent impressions of outcome, time to recovery, and factors predicting recovery. To do so, we chose CGI-I score as the primary outcome measure to incorporate patient and guardian impressions of recovery. This scale is recommended for use by the FND Core Outcome Measures group (
17) and allows for more emphasis to be placed on disability associated with tics rather than the features of tics themselves.
Rapid improvement was more likely for older patients and patients with a shorter time between onset and diagnosis. The importance of early diagnosis in prognosis in FND has been well established (
13), and our data provide additional support for this notion in the context of pediatric functional tics. Alternatively, the critical factor may be that a patient who experiences symptoms for a shorter duration at the time of diagnosis may be more likely to improve spontaneously. As many of our patients improved soon after the diagnosis of functional tics, this may highlight the importance of clear communication of the diagnosis early in the disease course. Most of our patients agreed with the diagnosis, which may have contributed to their overall improvement. In addition to patient education, ongoing treatment of functional movement disorders may include cognitive-behavioral therapy (
18) and physiotherapy (
19). Although data are lacking, comprehensive behavioral intervention for tics may be considered for treatment of functional tics given the similarity of the element of motor retraining used in physiotherapy for other functional movement disorders (
1,
6).
Our finding of an association between younger age and delayed recovery could indicate a difference in the association between age and prognosis in pediatric FND populations compared with adult FND populations. Studies evaluating adults with FND have noted an association between earlier age at onset and improved prognosis (
13). This relationship is consistent with improved prognosis in pediatric FND (
14,
15), including findings in Howlett et al.’s study of pandemic-associated functional tics (
16). The influence of age on FND outcome within pediatric populations is less well studied. Although age did not influence prognosis in Raper et al.’s analysis of pediatric FND (
15), we found that younger age was associated with lower likelihood of improvement at 1 month after diagnosis. Given the lack of power to correct for multiple comparisons, this finding requires replication. However, one possible explanation of this finding is that a greater ability to understand the diagnosis of FND with cognitive maturity improves prognosis.
Although the finding was not statistically significant, compared with their guardians, the patients tended to report more lingering or severe long-term symptoms. Because difficulty diverting attention from symptoms is a component of FND pathophysiology (
20,
21), patients may report more symptoms than their guardians. This potential relationship bears confirmation with further study.
Several recent reports of functional tics have noted associations with observing tics and tic-like movements on social media (
12,
22). However, only 50% of our cohort reported viewing tics on social media; others reported personal contacts with tics and sometimes stated that they “caught” tics from close contacts. This finding suggests a possible role for sociogenic elements outside of social media.
The features of tics noted in our patients were consistent with previous descriptions of functional tics and those observed by others during the COVID-19 pandemic (
9,
12,
23). As in any study of functional movement disorders, the distinction of functional from primary tic disorders is challenging (
23), and we cannot exclude the possibility that some of our cohort had a primary tic disorder, particularly because 13% of our cohort reported a family history of tics.
Many patients with FND are lost to follow-up. Therefore, a strength of our study is that follow-up data, in the form of a clinical encounter or a study interview, were available for all patients. A weakness of our study is the small sample size, which did not provide the power to correct for multiple comparisons. Additionally, some phenotypic features may have been present but not documented in the EMR, and therefore, the frequency of particular features may have been underreported. Data abstraction from EMRs was not blinded to the study hypotheses, which could have biased the interpretation of EMR-based estimation of CGI-I scores. Certain elements of our study, such as measures of patient-reported time to recovery, may be prone to recall bias; we sought to address this by framing most questions in relation to current symptoms.
Conclusions
In our cohort of pediatric patients with functional tics, rapid but incomplete recovery was common. We found support for the importance of early diagnosis in the treatment of functional tic disorders and raise the possibility that younger age may be a poor prognostic factor among pediatric functional tic populations.
Acknowledgments
The authors thank Mark Quigg, M.D., for providing formative feedback.