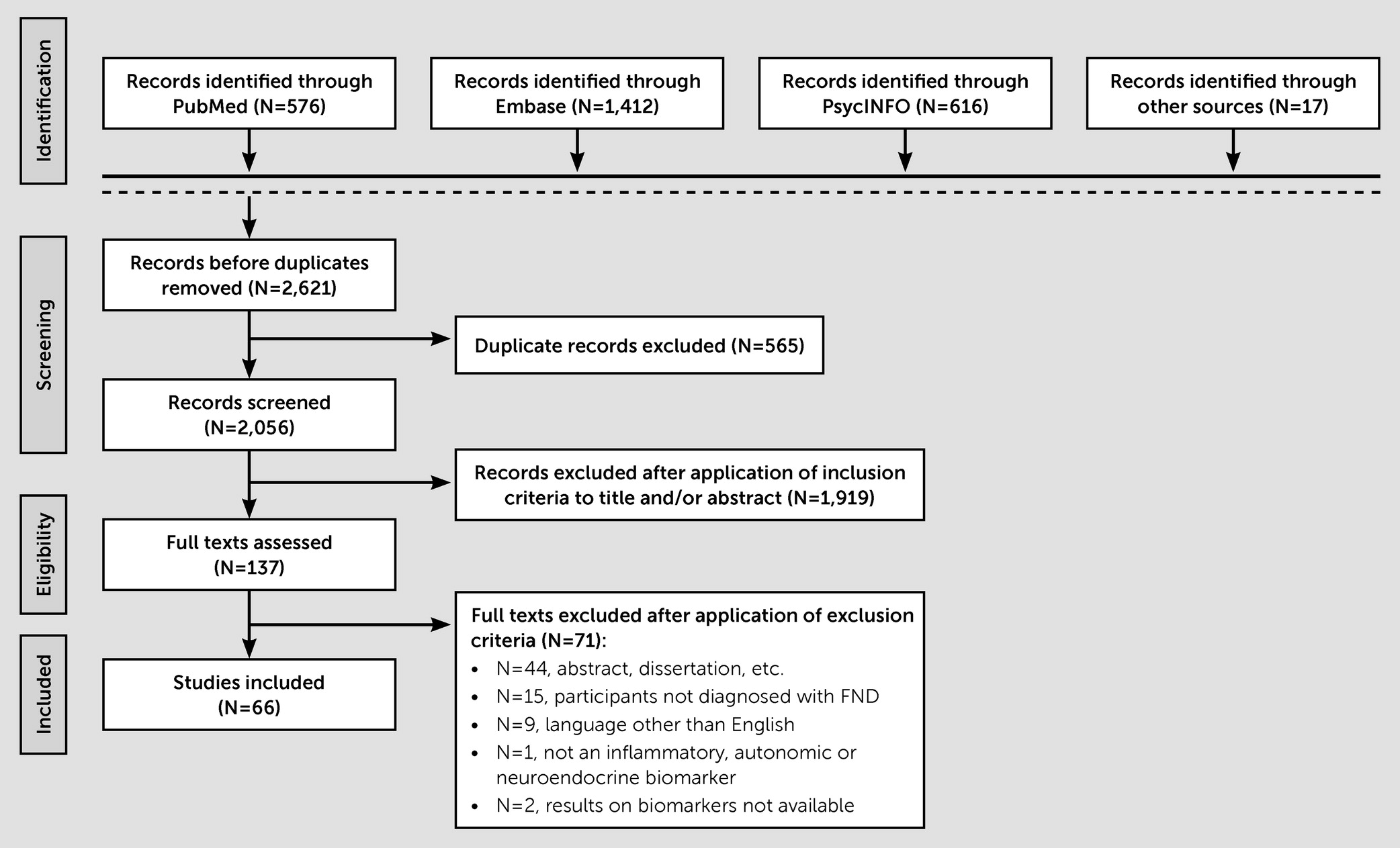
Functional neurological disorder (FND) is a condition at the intersection of neurology and psychiatry (
1). While of interest to early clinical neuroscience leaders, such as Jean-Martin Charcot and Sigmund Freud, FND was neglected by clinicians and academics alike throughout much of the late 20th century. Nonetheless, FND is the second most common condition seen in outpatient neurology clinics, a finding compounded by observations of high health care costs and poor prognoses among many patients (
2–
4). Renewed interest in the field has been promoted by recognition that FND can be reliably diagnosed using physical examination signs and semiological features (
1,
5). This high diagnostic specificity has led to a surge in research on the pathophysiology of FND, particularly using brain imaging approaches (
6,
7). Promising yet inconsistently identified neural circuit profiles have been observed to date (
6), suggesting that there are important gaps in our systems-level understanding of this condition. Thus, there is a need to contextualize autonomic, endocrine, and inflammation findings for a more complete mechanistic understanding of FND. Such efforts offer the promise of developing novel biologically-informed treatments.
Some functional neuroimaging studies across the motor spectrum of FND (including functional seizures [FND-seiz]) have identified several noteworthy findings in patients compared with healthy control subjects (HCs): enhanced amygdala reactivity in response to affectively valenced stimuli (
8,
9), increased task and resting-state connectivity between salience network and motor control circuits (
9–
11), and hypoactivation and altered connectivity of the right temporoparietal junction and inferior parietal lobule (
12–
14). Additionally, resting-state functional connectivity studies have found positive correlations between salience network and motor control network connectivity strength and patient-reported symptom severity (
11,
15). However, findings have been inconsistent across studies. For example, not all FND cohort studies have observed increased coupling between salience and motor control networks (
16).
Several gray and white matter characterization studies in FND samples have also implicated brain networks similar to those identified using functional neuroimaging, including two studies reporting that decreased white matter integrity of the stria terminalis and fornix (an amygdala outflow tract) correlated with FND illness duration and age at onset (
17,
18). Nonetheless, structural neuroimaging findings have also been inconsistently identified across studies (
6). Thus, while the neuroimaging literature suggests that some patients with FND have functional and structural alterations in brain areas implicated in emotion and threat processing, salience, arousal, agency, and motor control, the observed heterogeneity limits conclusions about specific FND neural signatures. This may relate to differences in disease-related mechanisms across patients, phenotypic heterogeneity, concurrently present neuropsychiatric disorders, medication effects, or compensatory neuroplastic effects.
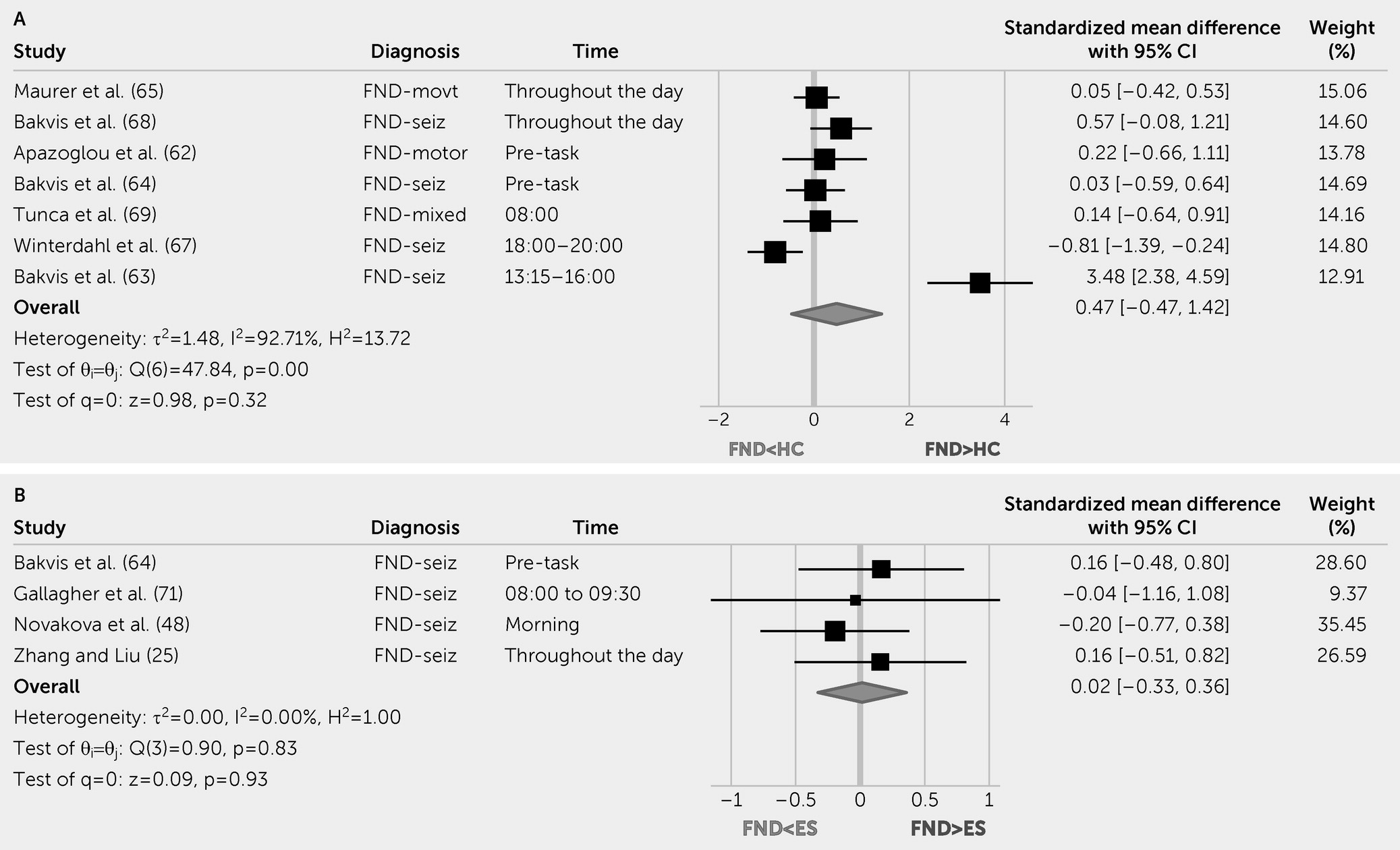
Given salience and limbic network involvement in some patients with FND, characterizing sympathetic or parasympathetic activity (e.g., heart rate [HR], HR variability [HRV], and skin conductance;
Table 1) and endocrine hypothalamic-pituitary-adrenal (HPA) axis markers are important gaps in the literature. Similarly, quantifying inflammation profiles, which are known to modulate salience and limbic networks (
19,
20), would add mechanistic value. In the present study, we conducted a systematic review and meta-analysis to comprehensively characterize the available literature on the autonomic, endocrine, and inflammation profiles of patients with FND. In addition, we contextualized this literature with other available pathophysiology considerations.
DISCUSSION
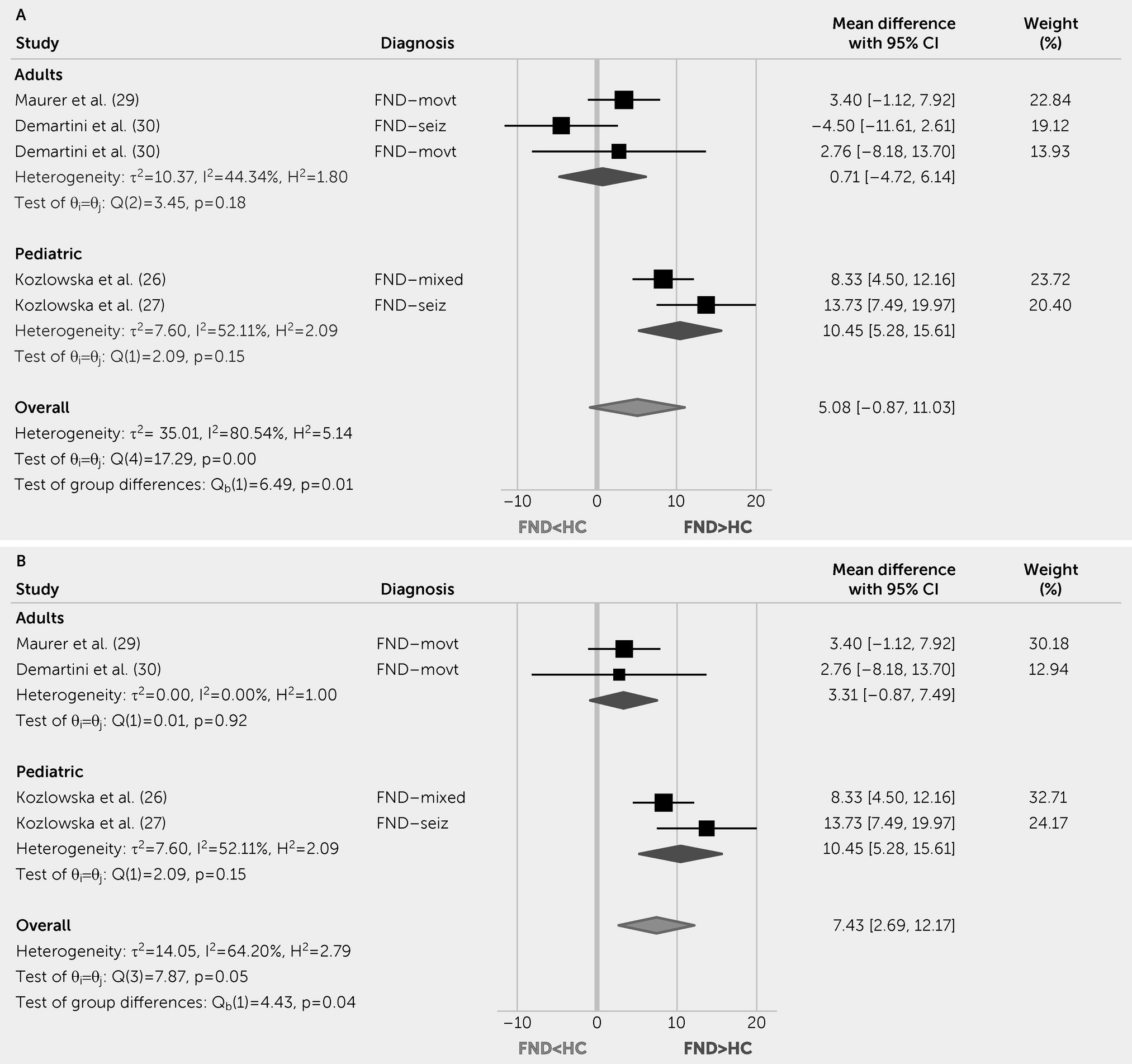
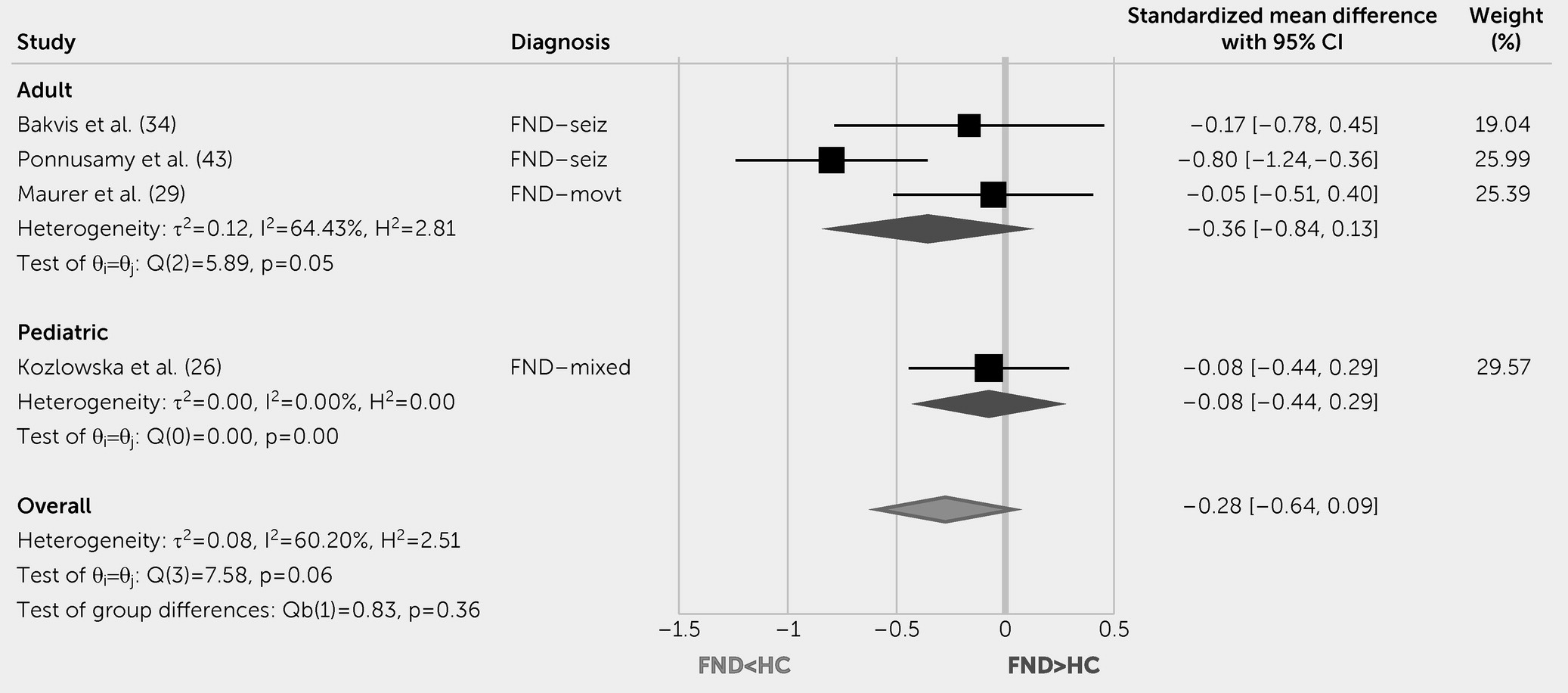
This systematic review and meta-analysis indicate that pediatric patients with FND compared with HCs have increased resting HR and lower parasympathetic tone (rMSSD levels). Autonomic studies in adults with FND yielded mixed results; initial evidence showed increased baseline HR compared with HCs, decreased peri-ictal HR versus epileptic seizures, and differentiating HR patterns on tilt table testing for functional syncope. Although other autonomic and endocrine measurements did not reliably differentiate patients with FND from control subjects, individual differences in these indices related to clinically meaningful variables. For example, autonomic measurements related to illness duration (
31), symptom severity (
31), perceived stress (
35), and mood or anxiety scores (
53,
54,
60) in patients with FND. In terms of inflammation data, this literature is in its early stages.
Evidence suggests that children and adolescents with FND have increased autonomic arousal, a finding that advances our pathophysiological understanding of FND in this subgroup (
89). Compared with HCs, pediatric FND showed increased HR and decreased HRV (rMSSD) at baseline and during cognitive and emotional tasks (
26–
28,
41). This suggests that compared with the prominent heterogeneity in adults with FND, autonomic markers reflecting increased sympathetic and decreased parasympathetic tone are more consistently present in this subpopulation. In one of the few studies analyzing neural circuit and autonomic data concurrently, Kozlowska et al. (
28) showed that heighted arousal (indexed using HR values) served as a moderator of increased delta power in salience and default mode networks in patients with pediatric FND compared with HCs (
28). Nonetheless, analyses also identified that autonomic activation patterns varied by age, attachment patterns, and clinical phenotype in children and adolescents with FND (
26). These differences underscore the presence of heterogeneity even in pediatric FND, pointing out that there are likely multiple mechanistic pathways implicated in this population (
89). Moving forward, it will be important to incorporate the diathesis-stress model of FND with developmental trajectories through the use of longitudinal studies (
90). In the pediatric literature, more work is needed to directly compare children and adolescents with FND to neuropsychiatric control subjects, including individuals with chronic pain, anxiety, and personality disorders, to evaluate the specificity of these promising results.
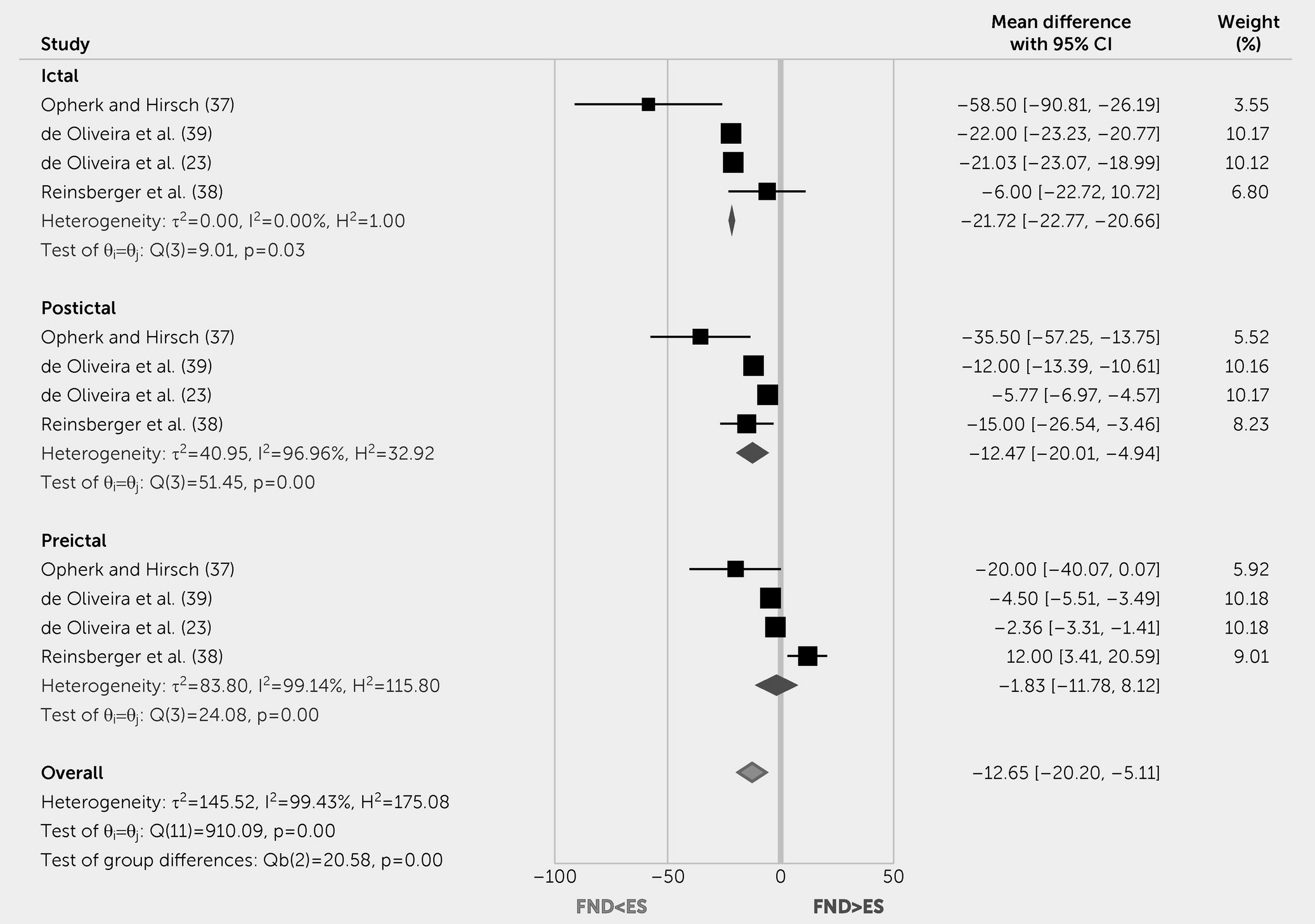
The systematic review and meta-analysis findings also indicate that peri-ictal HR levels differentiated patients with FND-seiz from those with epileptic seizures. Specifically, patients with epileptic seizures showed increased HR during the ictal and postictal periods. Other autonomic markers such as SCA and SCR yielded similar results; however, more studies are needed to determine the effect of FND-seiz on these physiological parameters. Nevertheless, the already identified autonomic peri-ictal differences between FND-seiz and epileptic seizures are noteworthy, particularly given the multiyear delay between symptom onset and diagnosis in patients with FND-seiz. While the capture of typical events on video-electroencephalogram remains the gold standard for diagnosis (
5), newly developed technologies can simultaneously measure HR, HRV, and SCA data, offering the opportunity to study the utility of composite diagnostic biomarkers in ambulatory and inpatient settings. Such an approach could help reduce the long diagnostic delays that are unfortunately far too common in this population. This wearable technology could also be extended beyond the spectrum of FND-seiz to aid the real-world capture of autonomic data across the full range of patients with FND.
The lack of high-quality studies investigating inflammation profiles in FND is an important gap in the literature, noteworthy given growing evidence showing a role for low-grade inflammation in the biology of a range of neuropsychiatric symptoms. For example, in a study investigating the biological and neurocognitive effects of a transient inflammatory response (i.e., typhoid vaccination) in heathy participants, self-reported fatigue correlated with activation changes in the posterior/mid insula and anterior cingulate cortex (
20). In a separate study using the same paradigm, inflammation-associated mood changes correlated with decreased cingulate gyrus-amygdala connectivity (
19). Furthermore, the combined influences of low-grade inflammation and perceived stress increased the risk of developing posttraumatic stress disorder (PTSD) in traumatized participants through disruptions in salience, default mode, and central executive network connectivity (
91). Given that the onset of FND can be precipitated by physical injury, surgeries, or infections (
1,
5) (processes that themselves are associated with inflammatory states), it will be impactful to further investigate possible associations between inflammation, brain networks, symptom severity, and prognosis across FND populations.
The heterogeneous findings identified across autonomic, endocrine, and inflammation data described in this article underscore the mechanistic, etiological, and methodological challenges of pathophysiology research in FND. To help illustrate the mechanistic complexity, an example is the role of the amygdala in this condition. Some studies have identified amygdala hyper-reactivity to affectively valenced stimuli, with time course data indicating impaired habituation (
9) and heightened sensitization (
8). However, using similar paradigms, others have reported no group-level differences or amygdala hypo-activations in patients with FND compared with HCs (
35,
92). Notably, the amygdala has afferent connections to the hypothalamus, dorsal motor nucleus of the vagus, and periaqueductal gray that drive sympathetic and parasympathetic tone and stress-related hormonal responses. What remains unclear in patients with FND is whether amygdala hyperactivity is coupled with parallel heightened autonomic and endocrine (i.e., cortisol, amylase) profiles, or if there is a mismatch between neurocircuit profiles and downstream responses. This knowledge gap is important because it is possible that heightened amygdala activity could reflect a relative inefficiency in appropriately activating fight-or-flight and other defense response pathways. In support of the latter possibility is evidence that illness duration is associated with reduced integrity of amygdala-based white matter pathways (
17,
18). Conversely, patients with FND have also demonstrated increased information flow (link-step connectivity) between the laterobasal (sensory) amygdala and the periaqueductal gray, suggesting that a limbic fast track in patients with FND may prime the nervous system for enhanced sympathetic responses (
11).
Mechanistically, another concern in FND research is whether disease-related neurobiological processes are shared or distinct across FND subpopulations (e.g., FND-seiz compared with FND-movt). In support of a transdiagnostic approach are clinical observations that many patients experience mixed symptoms, and yet others presenting with one phenotype develop distinct functional neurological symptoms. Notably, imaging profiles correlated to symptom severity in a mixed FND cohort remained significant after adjusting for subtypes (
11). Nonetheless, these questions remain actively debated, with some suggesting that FND-seiz should be considered a distinct entity from FND-movt (
93).
Etiological heterogeneity likely also contributes to heterogeneous findings described across autonomic and endocrine data. For instance, stressful life events and early life maltreatment are well-known predisposing vulnerabilities for FND (
94), with studies identifying that hypercortisolism was associated with trauma burden (
62,
68). However, antecedent adverse life experiences were not systematically accounted for across studies in this review, and some patients with FND do not report a history of traumatic life events; differences in trauma burden across FND samples could be a major factor contributing to inconsistently identified results. Such themes raise the question of a possible trauma-subtype of FND (
95).
Regarding methodological challenges, the multiplicity of additional physical symptoms (e.g., pain, fatigue) and commonly co-occurring neurological (e.g., traumatic brain injury) and psychiatric conditions (major depression, PTSD) that are themselves associated with altered autonomic, endocrine, and inflammation profiles are additional important considerations. For example, pain is a highly prevalent symptom in many patients with FND that is associated with a poor prognosis (
4); stratifying FND patients by the presence or absence of prominent pain would likely help disentangle the heterogeneity present in the literature (
96). Notably, pain is associated with heightened autonomic activity, increased inflammation, and endocrine alterations in other neuropsychiatric conditions (
97). Similarly, PTSD is associated with hypercortisolism and elevated inflammation markers (
98). To adequately account for these factors and other confounds (e.g., medication effects, time and type of cortisol sample drawn), larger samples are undoubtedly needed. Larger patient samples would also assist in comprehensively investigating the complex interplay between functional neurological symptoms and behavioral, psychological, autonomic, endocrine, inflammation, neuroimaging, and epigenetic and genetic data (
99). Furthermore, replication of the same methods across studies need to be encouraged to allow for straight forward comparisons of results. More research is also needed to determine to what extent FND is mechanistically (and etiologically) similar or distinct across phenotypes. Lastly, given that several negative physiology studies found differences in self-report measures, as well as in cognitive and behavioral task performances, it is worth considering the overall utility (and framing) of biomarker research in this field.
In summary, the study of autonomic, endocrine, and inflammatory measurements in patients with FND remains a promising, yet complex, area of research. Given that FND is likely mechanistically and etiologically heterogenous, the study of biologically informed subtypes and composite biomarkers may be particularly fruitful future directions.