Patients with psychotic disorders are treated primarily in public mental health settings such as community mental health centers and state hospitals. A comorbid substance use disorder, a poorly controlled medical illness, or psychotic symptoms associated with disorders other than schizophrenia and schizoaffective disorder are common among these patients (
1,
2,
3). Community mental health centers typically provide services that affect outcomes for patients with psychotic disorders, such as case management and rehabilitation (
4,
5). However, few trials of novel antipsychotic medications have taken place in these settings (
6,
7,
8), and therefore the effectiveness of such medications in public mental health settings remains unclear (
9).
Poor psychosocial functioning and a low likelihood of benefiting from rehabilitation are particularly associated with negative symptoms of psychotic disorders (
10,
11). Novel antipsychotics such as olanzapine have been shown to be efficacious in reducing negative symptoms (
12,
13,
14,
15), which raises the question of whether these medications will act synergistically with rehabilitative treatments to improve outcomes (
16).
We monitored a consecutive series of patients at a community mental health center who switched to olanzapine treatment and a group who continued to take conventional antipsychotics. The goals of this study were to evaluate the effectiveness of switching to olanzapine for patients in such a setting and to explore whether olanzapine acted in concert with psychosocial rehabilitation to improve outcomes.
Methods
Sample
The study took place at a community mental health center that has an international reputation for developing and implementing cutting-edge case management and psychosocial rehabilitation interventions (
17,
18,
19). Patients receive continuous clinical case management services that follow National Institute of Mental Health guidelines (
20). Assertive community treatment teams are available for patients with high service needs (
21).
All patients at the center are encouraged to participate in psychosocial rehabilitation interventions, which are an integral part of the community support program. Rehabilitation interventions include the individual placement and support approach to vocational rehabilitation (
22), social skills training modules (
23), and integrated substance abuse treatment (
24,
25). Case managers are responsible for identifying functional impairments, designing a treatment plan in collaboration with the patient and his or her psychiatrist, and encouraging follow-through.
Potential participants for this study were identified by case management teams that serve patients with psychotic disorders. Depending on what medications they were taking, patients were assigned to the olanzapine group or to the reference group. The participants' symptoms and psychosocial functioning were rated prospectively. Treatment was naturalistic and not controlled for the study. Continuation and dosing of the primary antipsychotic and any concurrent medications were at the discretion of the patients and their psychiatrist. All the study participants met the diagnostic and functional impairment criteria for severe mental illness set by the New Hampshire Division of Behavioral Health. Both groups of patients received case management and rehabilitation services throughout the course of the study.
The olanzapine group comprised a consecutive series of patients who switched from another antipsychotic medication to olanzapine. Baseline data were those collected immediately before patients were started on olanzapine. When there was an overlap between the previous medication and olanzapine, the baseline point was defined as the date when olanzapine was initiated. A subset of the ratings was repeated at three months, and all ratings were repeated after six months of treatment.
A total of 109 patients who began olanzapine treatment between October 1996 and April 1998 gave informed consent to be monitored for this study. Five patients (4 percent) stopped taking olanzapine before the first follow-up assessment (
33). The 104 remaining patients constituted the olanzapine group; one patient switched to risperidone after three months, and 103 continued a trial of at least six months. The mean daily dose at six months was 15.28 mg (median=15 mg, mode=20 mg, range=5 to 40 mg).
The reference group comprised 49 patients who were treated with conventional antipsychotics and who gave consent between December 1996 and July 1998 to enter the study. At six-month follow-up, two patients had discontinued their medication, and one had switched to olanzapine. Thus, 46 patients (94 percent) remained on conventional antipsychotic treatment throughout the study period. Their mean daily chlorpromazine-equivalent dose at six months was 393.11 mg (range=10 to 1,750 mg).
Four patients who terminated olanzapine trials within a few days were monitored in the reference group in an attempt to maximize the equivalence of the two groups. We collected the same baseline data on the reference group patients at the point of consent and monitored them with the same protocol for six months.
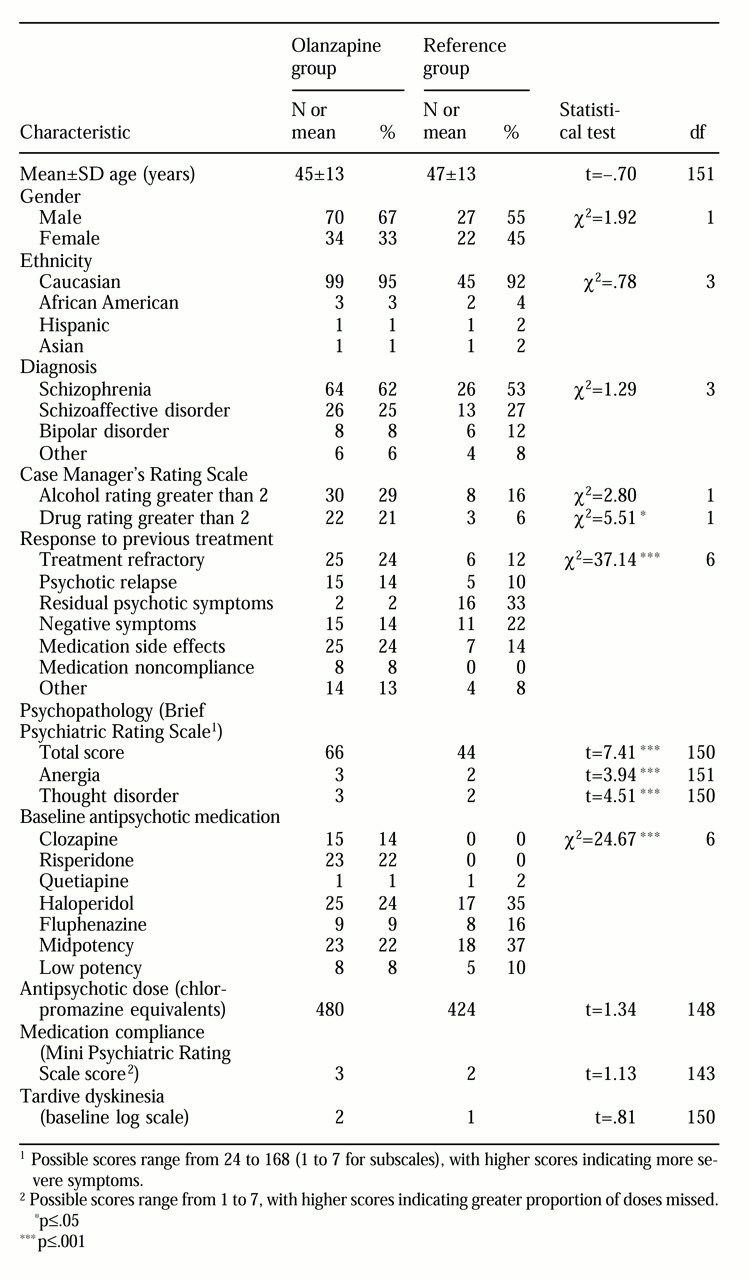
Table 1 summarizes the demographic and clinical characteristics of the two groups. Age, gender, ethnicity, diagnosis, tardive dyskinesia history, mean chlorpromazine-equivalent dose, and severity of medication noncompliance did not differ significantly between the two groups at baseline. Patients in the olanzapine group were significantly more likely to be symptomatic, to abuse drugs, and to have treatment-refractory illness or to be treatment intolerant at baseline. Baseline antipsychotic treatment differed as well, with the olanzapine group more likely to have been taking clozapine or risperidone.
Assessments
An antipsychotic treatment log for each participant was completed at baseline, at three months, and at six months. The baseline log identified demographic characteristics, diagnosis, current antipsychotic treatment, concurrent psychiatric medications, and the treating psychiatrist's ratings of tardive dyskinesia history and response to previous treatment. Psychotic relapse was defined as an exacerbation of psychotic symptoms following a period of clinical stability. Clinical diagnoses were confirmed during the baseline interview using
DSM-IV criteria. Chlorpromazine-equivalent dose for antipsychotics was calculated following Mason and Granacher (
26). For atypical antipsychotics, on the basis of extrapolations from efficacy trials comparing these agents to conventional antipsychotics (
12,
13), we calculated 100 mg of chlorpromazine to be equal to 100 mg of clozapine, 2 mg of risperidone, and 100 mg of quetiapine.
The baseline and follow-up logs included scores on the Clinical Global Improvement (CGI) scale and a condensed version of the Brief Psychiatric Rating Scale (BPRS) entitled the Mini Psychiatric Rating Scale (MPRS). The seven-item MPRS was created to allow a rapid rating of clinical state. The positive symptoms item merges the hallucinations and unusual thought content items from the BPRS. The negative symptoms item merges the blunted affect and emotional withdrawal items from the BPRS with apathy and anhedonia descriptors from the Scale for Assessment of Negative Symptoms (
27). The anxiety, depression, and disorganization items are identical to the corresponding items in the BPRS. Two new items were created following the BPRS format; one rates the severity of medication side effects and the other rates medication noncompliance.
The standard 24-item BPRS and an expanded version of the Case Manager Rating Scale (CMRS) (
1,
29,
30) were administered at baseline and at six-month follow-up. The CMRS was developed in the 1980s to capture case managers' detailed knowledge of the symptoms and psychosocial functioning of their patients in the community who had severe mental illness (
28). The original descriptors were modified to focus on functioning rather than on supports, and several new items were added to yield the CMRS-Plus. The well-validated substance abuse items were not altered (
1).
The CMRS-Plus has two sections. The 12 items in the psychosocial functioning section are rated from 1 to 5—highly functional to highly impaired. Items cover vocational functioning, family relationships, social relationships, grooming, physical activity, meals, finances, and criminality. The 17 items in the illness factors section are rated from 1 to 5—not present to extremely severe—except for the alcohol use and drug use items, which correspond to DSM-IV criteria. Other items include psychotic symptoms, negative symptoms, suicidality, hostility, and abnormal movements.
The CMRS-Plus was completed by the patient's case manager according to his or her composite knowledge of the patient over the previous three months. All other ratings were made by a research psychiatrist on the basis of a direct interview.
Analysis
Baseline characteristics of the two groups were compared. For continuous variables, we used Student's t tests. For categorical variables we used chi square tests unless cell sizes were too small, in which case we used Fisher's exact test.
Because the reference group was not an experimental control group formed by random assignment, we contrasted the outcomes of the olanzapine group with both their own baseline status and with the outcomes of the reference group. For the within-group comparison for the olanzapine group, we used repeated-measures paired t tests. For the between-group comparisons we used repeated-measures analysis of variance. We repeated the analysis of variance after removing data for patients who were rated as being in psychotic relapse on the baseline log to evaluate for bias resulting from regression toward the mean among these patients (
31).
Results
Olanzapine group pre-post comparison
The outcomes of the patients in the olanzapine group are summarized in
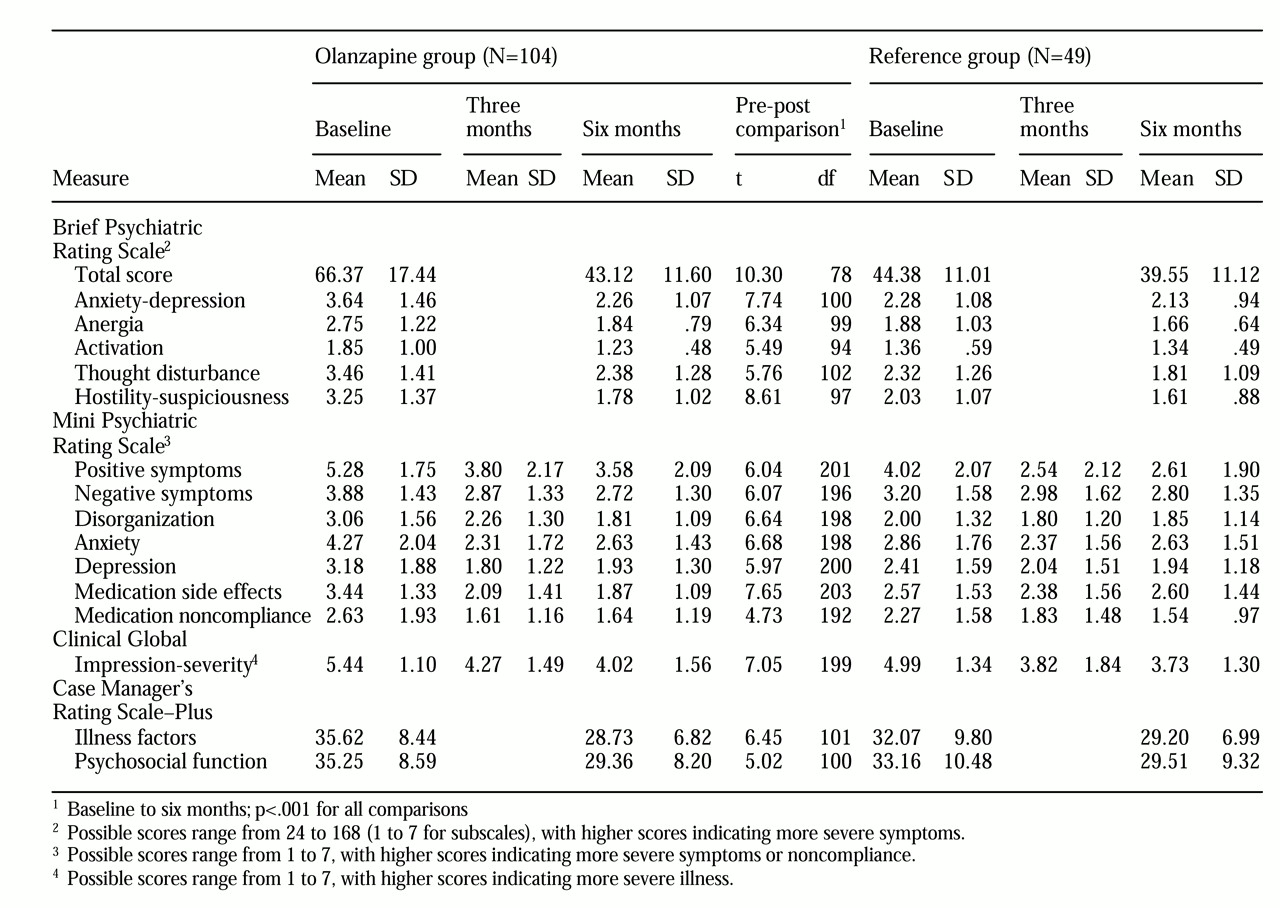
Table 2. First we compared the patients' outcomes at six months with their own baseline status. The mean total BPRS score for the group was 66.37 at baseline before olanzapine was prescribed; the mean score fell to 43.12 after six months on the medication. As the minimum score on the BPRS is 24, this represents a reduction of 55 percent in mean BPRS total score. The group demonstrated significant improvement on each BPRS subscale as well.
Analysis of the MPRS items revealed that the group demonstrated significant improvement on each item by the three-month follow-up (positive symptoms, t=5.31, df=201, p<.001; negative symptoms, t=5.35, df=196, p<.001; disorganization, t=4.28, df=198, p<.001; anxiety: t=8.02, df=198, p<.001; depression, t=6.66, df=200, p<.001; medication side effects, t=6.59, df=203, p<.001; medication noncompliance, t=4.90, df=192, p<.001). Only on the disorganization item was there a significant further reduction in patients' mean scores from three-month to six-month follow-up (t=2.42, df=198, p=.016). Six-month outcomes were superior to baseline on all items (
Table 2).
The treating psychiatrists rated the group as less severely ill on the CGI severity subscale by three-month follow-up, with minimal further improvement by the six-month point. The scores correspond to a rating between markedly ill and severely ill at baseline, with improvement to moderately ill at three months and at six months. On the CGI improvement subscale, patients were rated by their psychiatrists as having demonstrated between minimal improvement to much improvement at both three-month and six-month follow-ups.
Case managers also rated the group's symptoms as significantly improved. CMRS-Plus scores on illness factors dropped from a mean of 35.6 at baseline to 28.7 at six-month follow-up, a 37 percent reduction. Scores on substance abuse items indicated significant improvement as well. Among 30 patients with active alcohol abuse or dependence at baseline (CMRS-Plus alcohol use item greater than 2), the alcohol use rating fell from a mean of 3.43 at baseline to 2.37 at follow-up (t=4.62, df=29, p<.001). Among 22 patients with active drug abuse or dependence at baseline, the drug use rating fell from a mean of 3.59 at baseline to 2.31 at follow-up (t=4.22, df=21, p=.001).
The olanzapine group's functional outcomes also improved significantly. On the psychosocial function scale of the CMRS-Plus, the mean score dropped from 35.3 before olanzapine was prescribed to 29.4 at six months, which represents a 25 percent improvement in overall functional ability.
Olanzapine group versus reference group
We used analysis of variance to compare the outcomes of the olanzapine group and the reference group. The results are summarized in
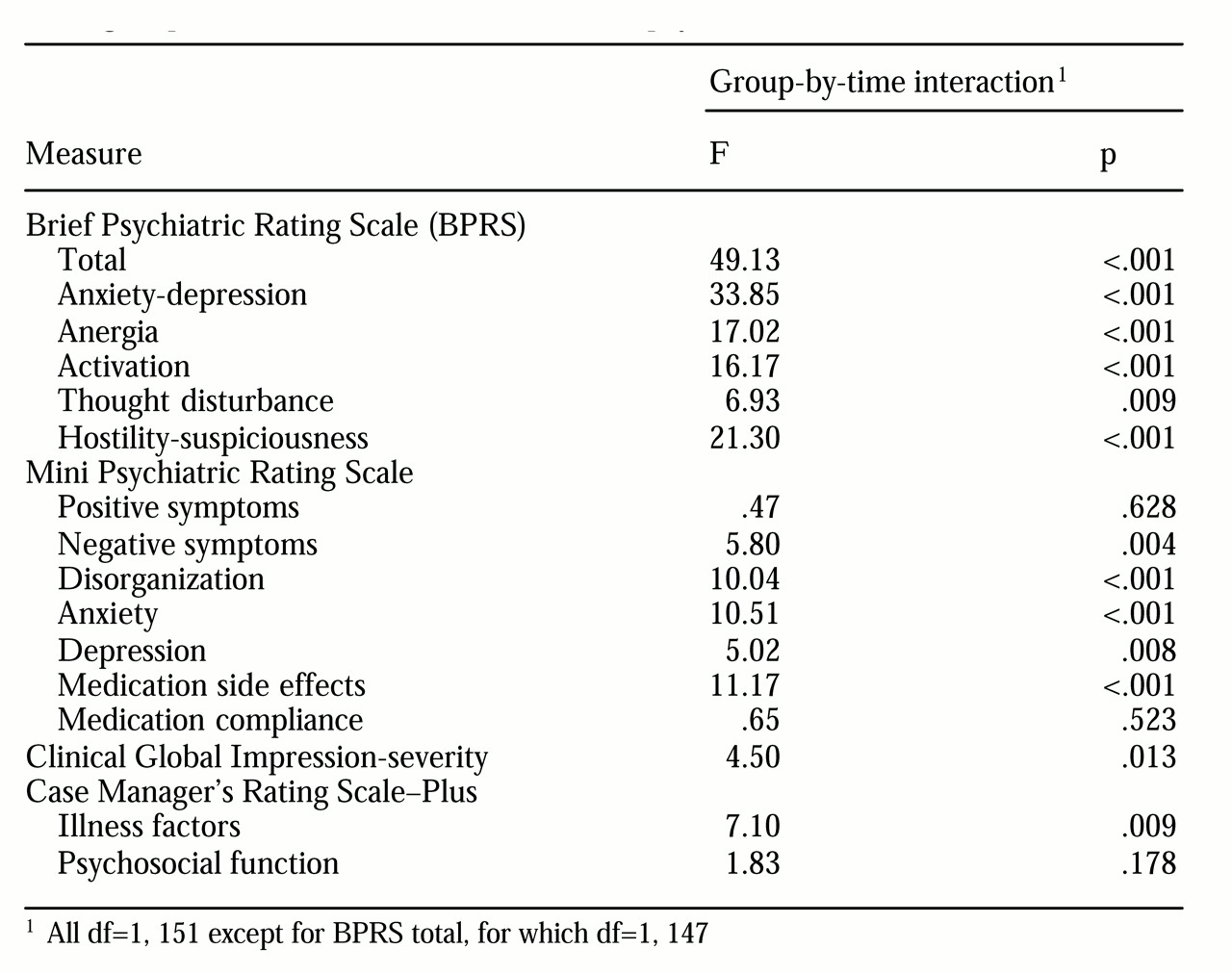
Table 3. A significant group-by-time interaction in mean BPRS total score indicated that the olanzapine group had greater improvement in symptoms over six months than the reference group. The olanzapine group also demonstrated greater improvement on each BPRS subscale.
On the MPRS, the positive psychotic symptoms and medication noncompliance items improved at a similar rate in both groups. However, the olanzapine group had superior improvements in negative symptoms, disorganization, anxiety, depression, and medication side effects.
On the CGI severity scale, the olanzapine group was rated as more severely ill overall and demonstrated significantly larger reductions in severity over time. On the CGI improvement subscale, the olanzapine group was rated as having had significantly greater improvement than the reference group at both the three-month follow-up (F=17.88, df=1, 150, p<.001) and the six-month follow-up (F=17.36, df=1, 150, p <.001).
On the CMRS-Plus illness factors scale, the olanzapine group demonstrated greater improvement than the reference group. The substance use items were examined in detail among patients with an active substance use disorder at baseline. On the alcohol use scale, 30 olanzapine patients and eight reference group patients met criteria (CMRS greater than 2). Both groups had significant improvement at six months (F=28.67, df=1, 36, p< .001), but no difference was observed in the rate of improvement between the two groups. On the drug use scale, 22 olanzapine patients and three reference group patients met criteria. Again there was improvement in both groups over time (F=22.54, df=1, 23, p<.001), but the number of patients in the reference group was too small for a valid comparison.
On the CMRS-Plus psychosocial function scale both groups had significant improvement over six months (F=47.89, df=1, 151, p<.001). The difference in the rate of improvement between the two groups did not achieve statistical significance, although there was a trend in favor of the olanzapine group.
We repeated the analyses of variance after removing data for patients who were in psychotic relapse at baseline. A total of 89 patients were left in the olanzapine group and 44 in the reference group. The difference in improvement in psychosocial functioning measured on the CMRS-Plus then achieved statistical significance, indicating greater improvement in the olanzapine group (F=3.89, df=1, 131, p=.051). All other findings remained the same.
Discussion
Caution must be exercised when interpreting the results of this study. All of the participants were receiving continuous case management and rehabilitation in addition to medications, and they would be expected to demonstrate some improvements over baseline with time alone. The two study groups were also clearly nonequivalent at baseline, even though they did not differ in demographic characteristics.
Because the olanzapine group was more impaired at baseline and the reference group demonstrated better response to previous treatment, different patterns of recovery would be expected. For example, "floor effects" could have kept patients in the reference group from achieving equivalent improvement if they started the study with ratings near the bottom of a scale. On the other hand, greater severity of symptoms among the patients in the olanzapine group could have hindered their progress in psychosocial rehabilitation.
The pattern that emerged across most symptom measures was greater symptom severity in the olanzapine group at baseline that dropped to a severity level similar to that of the reference group at three-month and six-month follow-ups. This pattern held across both reference points, multiple symptom measures, independent raters, and ratings based both on cross-sectional interviews and composite collateral information. The average reductions in symptom severity, ranging from 37 percent to 55 percent, indicated a clinically significant change in psychopathology. Therefore, we have substantial confidence that superior improvement in symptoms occurred in the olanzapine group. This improvement appears to be the result of large improvements among highly symptomatic patients after they switched to olanzapine, while patients who continuined with conventional antipsychotics improved only modestly.
These findings remained unchanged even after we excluded data for patients who were in psychotic relapse at baseline. Relapsing patients are measured at their worst at baseline, and they may show exaggerated improvements with time regardless of treatment (
31). The constancy of our findings indicates that the improvements in the olanzapine group were not merely caused by overrepresentation of relapsing patients. Therefore, it appears that as a result of the switch to olanzapine, patients with sustained high levels of symptom severity became relatively indistinguishable from a stable group of patients who did not require a medication change. In other words, patients with treatment-resistant illness became more responsive to treatment. These findings are consistent with previous findings (
33).
The MPRS interval ratings showed that although most symptom improvement had leveled off in the olanzapine group by three months, disorganization symptoms continued to improve. Therefore, disorganization symptoms may have a more protracted response to olanzapine than other symptoms. Longer-term follow-up is needed to see how much additional improvement in disorganization this group may achieve over time.
The significant improvements in both alcohol and drug use outcomes in the olanzapine group are the first to our knowledge to be reported with olanzapine treatment. The number of patients with active substance abuse in the reference group was too small to allow for meaningful comparisons between the two groups. Therefore, it is unclear whether the reduction in substance abuse resulted solely from substance abuse rehabilitation, which has been shown to be effective (
25).
Both groups showed a reduction in medication noncompliance. Some authors have speculated that the greater tolerability of atypical antipsychotics will lead to improved medication compliance (
34). Choice of medication may not affect medication compliance in the community, however, and both groups had relatively high medication compliance at baseline. The center at which this study was performed initiated an outreach team focused on medication monitoring in 1996 that may have had so strong an effect on medication compliance that differences between the groups were not evident.
In contrast, psychosocial functioning improved more consistently. The patients in the olanzapine group achieved a 25 percent improvement over their own baseline status and showed a trend toward greater improvement than the patients in the reference group. The olanzapine group began the study more functionally impaired and achieved six-month outcomes similar to those of the reference group. However, the olanzapine group also started with higher levels of negative and disorganization symptoms, which should have impeded progress in rehabilitation (
10). We were surprised to find that this difference achieved statistical significance when data for patients in psychotic relapse at baseline were removed. Acutely relapsing patients were overrepresented in the olanzapine group. Their exclusion may have eliminated statistical noise by achieving a more homogeneous group. Alternatively, relapse may interrupt progress in rehabilitation, delaying functional improvement for relapsing patients beyond six months.
Limitations of this study include the fact that treatment was not restricted; therefore some patients were taking medications besides their primary antipsychotic that may have contributed to their outcomes at six months. Also, the reliability and validity of the MPRS have not been tested, and the medication side effects and noncompliance items are newly created and are not duplicated in other measures. We could not distinguish the relative contributions of improvements in primary negative symptoms from reductions in extrapyramidal symptoms to lowering negative symptom scale scores, as previous studies have done (
15). Raters were not blinded to treatment condition, which could have biased their ratings.
Finally, this study used an effectiveness design, maximizing the external validity of the results but preventing measurement of cause-and-effect relationships between specific components of the treatment and the outcomes reported (
9).