In this study, we set out to determine whether extrapyramidal symptoms differed among neuroleptic-free elderly patients with DAT, major depressive disorder (MDD), and schizophrenia or delusional disorder (SCHIZ/DELUS). Multivariate statistical techniques were used to control for the effects of age and the severity of depressive or psychotic symptoms.
DISCUSSION
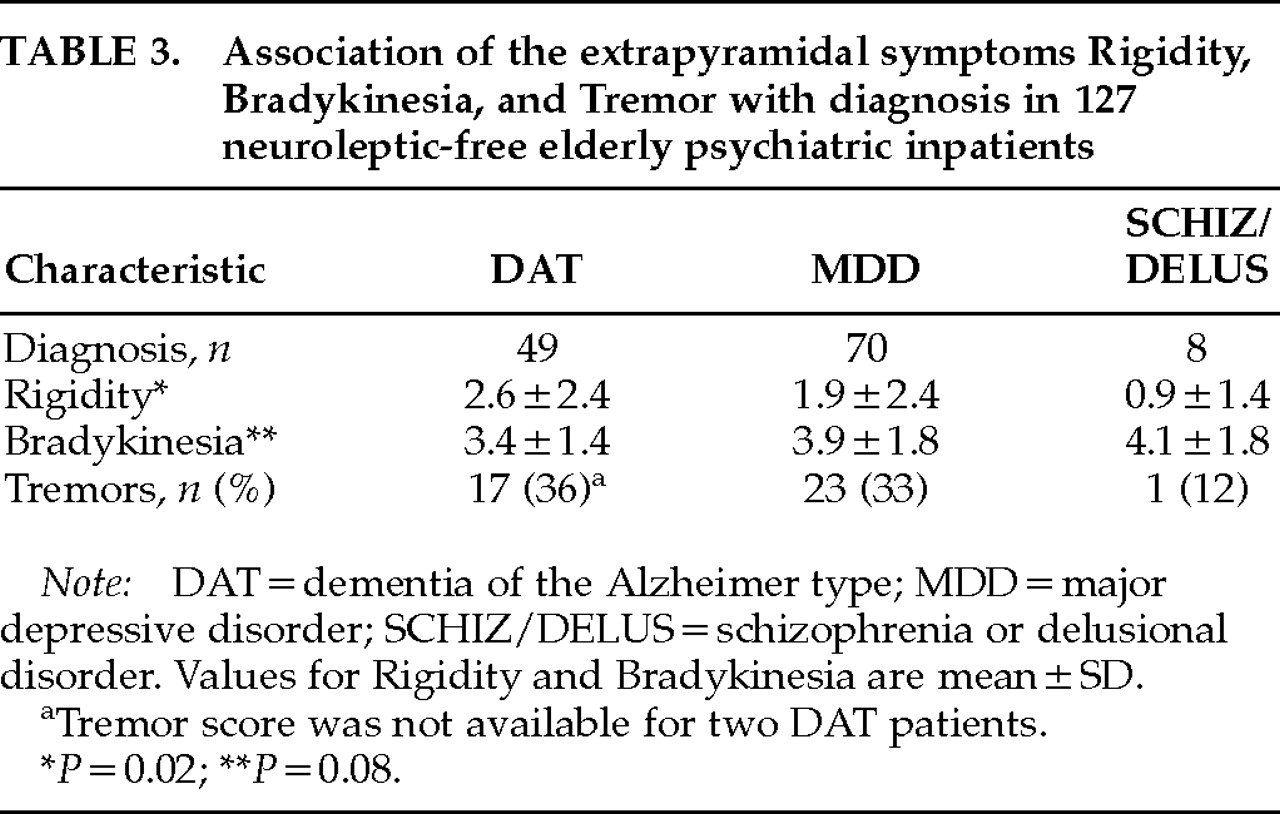
We compared the severity of three aspects of EPS—parkinsonian rigidity, bradykinesia, and tremor—in neuroleptic-free elderly psychiatric inpatients diagnosed with either dementia of the Alzheimer's type, major depressive disorder, or a psychotic disorder (schizophrenia or delusional disorder). Parkinsonian rigidity, but not bradykinesia or tremor, was independently associated with diagnostic group, with a rank order of severity of rigidity of DAT>MDD>SCHIZ/DELUS. Although not associated with diagnostic group, bradykinesia was positively associated with a measure of withdrawn depression and with male gender. No significant association was found between the presence of tremor and any of the identified variables.
We found significantly elevated ratings of parkinsonian rigidity in our DAT patients in comparison to the MDD and SCHIZ/DELUS groups, but bradykinesia and tremor did not differ among groups. Because we did not include normal elderly control subjects in the current study, we cannot conclude whether the degree of rigidity, bradykinesia, and tremor observed in our patients was abnormal for individuals of this age. Abnormalities of bradykinesia, however, have been reported in all three diagnostic groups relative to normal control subjects,
12,13,15,20,32 with abnormalities in parkinsonian rigidity and tremor reported for DAT and SCHIZ/DELUS patients.
2,15,20,32 The finding of a difference in Rigidity scores between our patients diagnosed with DAT and elderly psychiatric control subjects therefore extends these prior reports by suggesting that parkinsonian rigidity is specifically elevated in DAT and is not merely a nonspecific concomitant of aging or of late-life mental disorders. In contrast, these findings suggest that the reported increases of bradykinesia and tremor in DAT patients are not unique to this diagnosis, but may be mediated through mechanisms common among late-life neuropsychiatric illnesses.
We are aware of only one prior report that examined differences in spontaneous EPS between DAT and psychiatric patient groups; however, instrumental rather than clinical measures of rigidity, movement time (a measure of bradykinesia), and tremor were used. Caligiuri et al.
15 examined 13 elderly patients diagnosed with DAT and 13 diagnosed with schizophrenia. They found that only on the measure of movement time did the DAT patients show significantly more EPS than the patients with schizophrenia. Although the discrepancy between the findings of Caligiuri et al. and those of the present study, which found an independent association between psychiatric diagnosis and rigidity, but not bradykinesia, are not readily resolvable, they may be due to the different methodology of assessing EPS. Instrumental techniques for the measurement of EPS appear to be sensitive to subclinical abnormalities.
16,32 Thus, it is possible that instrumental measures might identify subtle between-group differences not appreciable on clinical evaluation (in bradykinesia, for example). Similarly, if, as a consequence of increased sensitivity, instrumental measures have a lower “ceiling” than clinical EPS measurement, the use of instrumental techniques might
not detect clinically observed differences between patient groups (in rigidity, for example). It is also possible that the discrepancy between the current findings and those of Caligiuri et al. might also result from the lack of generalizability attendant on the small number of SCHIZ/DELUS patients in both studies and the small number of DAT patients in the latter report. Because our finding of an association between diagnostic group and increased rigidity persisted when we limited our analysis to the larger DAT and MDD groups, however, the finding seems unlikely to have resulted from this form of a type I error.
We also examined whether the presence of EPS in our elderly psychiatric patients was due to an association with the severity of depressive or psychotic symptoms, rather than to an association with a diagnosis of a depressive or psychotic disorder per se. Thus, bradykinesia, although not demonstrating a specific association with a diagnosis of depression, was correlated with measures of depression across diagnostic groups. The severity of bradykinesia was positively correlated with the BPRS measure of withdrawn depression and, correspondingly, was negatively correlated with the BPRS measure of anxious depression. Although similar symptoms rated on the Ham-D (Ham-D-Anergic and Ham-D-Anxious) were not independently associated with bradykinesia, this is explained in part by the substantial covariance of the corresponding Ham-D and BPRS subscales (
r=0.6 and
r=0.8, respectively, both
P<0.001). We identified one prior study, limited to patients diagnosed with MDD, that reported on the relationship between EPS and severity of depressive symptoms. Rogers et al.
13 found no correlation between total Ham-D score and an instrumental measure of movement time, nor did change in total Ham-D score after treatment correlate with change in movement time. Subscales of the Ham-D were not examined, however, which leaves the possibility that any associations were obscured by the opposing contributions of anxious and anergic factors. Interestingly, measures of clinical EPS in their MDD patients did improve significantly with treatment of depression, although the authors did not report which specific EPS were improved or their correlations with the reduction in depressive symptoms. Taken with the current findings, this linkage is consistent with a common mechanism contributing to the expression of certain depressive symptoms and bradykinesia.
We also found an independent association between increased bradykinesia and male gender. Mean bradykinesia ratings in our male patients were 4.3±1.6, versus 3.5±1.6 in our female patients. Gender differences in severity of EPS have been previously reported in DAT,
33,34 with greater frequency of degenerative changes of the substantia nigra in male patients at postmortem examination.
35 Gender differences in the prevalence of idiopathic Parkinson's disease have also been reported.
36 Although several lines of preclinical investigation indicate that estrogens modulate dopaminergic neurotransmission,
37–40 this is unlikely to explain the observed difference in our elderly patients, since the females were postmenopausal and only infrequently received estrogen replacement therapy (unpublished data). Further exploration of the role of gonadal steroids in modulating dopaminergic neurotransmission and motor function in elderly populations, however, may be warranted.
Unlike our finding of an association between bradykinesia and withdrawn depression, we found no association between depression and either rigidity or tremor, nor between any EPS and measures of psychosis. This stands in contrast to reports of an association of EPS with psychotic symptoms in studies limited to DAT patients.
4,17 To examine this issue in our sample, we re-analyzed the association of psychotic symptoms with Rigidity, Tremor, and Bradykinesia in our DAT patients only; however, no significant relationships were found. In contrast, the observed correlations between bradykinesia and symptoms of depressive withdrawal remained significant within the DAT group (
r=0.3,
P=0.02). An important difference between the present study and the earlier reports of Gilley et al.
17 and Mayeux et al.
4 was the current use of patients presenting for acute psychiatric hospitalization for treatment of psychosis, depression, or other behavioral syndromes.
19 Thus, when we confined our analysis to DAT patients, we were contrasting our DAT patients with psychotic symptoms to our DAT patients without psychosis but with prominent symptoms of depression, irritability, or aggression. This may have limited our power to detect an association of EPS and psychosis, since agitation and irritability have also been associated with EPS.
4,41Although the specific mechanism underlying the association of EPS with DAT is not known, there is evidence to suggest that degradation of nigrostriatal dopamine pathways is in part responsible. Losses of 30% to 50% of dopaminergic neurons in the substantia nigra, with a 50% reduction of dopamine concentration in the striatum, have been reported in normal aging (for a recent review, see Palmer and DeKosky
42). Reductions in dopaminergic function beyond that seen with age alone have also been reported in DAT.
43–45 This pattern of degeneration of dopaminergic function parallels the clinical occurrence of EPS, which are more frequent in DAT patients than in normal elderly subjects, and more frequent in normal elderly than in normal young subjects.
10,20 A similar association between EPS and decreases in dopaminergic function has been reported within groups of patients diagnosed with DAT. Thus, Kaye et al.
47 reported cerebrospinal fluid homovanillic acid (HVA) concentrations in 10 DAT patients with EPS to be 40% of the corresponding values in 27 DAT patients without EPS. A decrease in the CSF concentration of biopterin (an enzyme cofactor for the rate-limiting step of dopamine synthesis) was also found. These differences remained significant after controlling for the effects of age and severity of cognitive impairment. Similarly, we found lower concentrations of plasma HVA to be correlated with increasing severity of parkinsonian rigidity in DAT patients.
48Findings from postmortem studies both support and extend the
in vivo data. Antemortem EPS in DAT patients are associated with postmortem evidence of reduction in dopamine concentration, HVA concentration, and number of dopaminergic neurons.
34,49,50 Antemortem EPS in DAT patients are also associated with postmortem evidence of cortical and subcortical Lewy bodies.
51,52 In the absence of a longitudinal design with neuropathologic confirmation of diagnosis, we cannot establish the frequency of SDLBT among our patients. Nor did we attempt to apply proposed diagnostic criteria for SDLBT
6 in an antemortem effort to identify the SDBLT patients among our DAT group. Current estimates of the frequency of cortical and subcortical Lewy bodies in patients receiving an antemortem diagnosis of DAT, however, range from 20% to 30% (for a recent review, see Ellis et al.
53), making it likely that a similar proportion of our DAT patients, and a larger percentage of our DAT patients with prominent EPS, would demonstrate Lewy bodies at postmortem exam. Whether Lewy body pathology contributes directly to the risk for EPS or is merely a marker for the associated degeneration of dopamine systems remains to be determined.
Determination of whether the differences in severity of EPS among DAT, MDD, and SCHIZ/DELUS patients observed in the present study also reflect progressive impairments in dopaminergic function requires direct comparison of neurochemical and neuropathological markers of dopaminergic function among these groups. Although a substantial number of studies have examined dopaminergic measures in schizophrenic patients (for review, see Csernansky and Newcomer,
54 Kahn and Davis,
55 or Friedhoff and Silva
56), evaluation of the relationship of these measures to EPS in unmedicated patients or comparisons with MDD and DAT groups are infrequent. A number of studies, however, have examined dopamine receptor density in either schizophrenic or DAT patients in comparison to age-matched normal control subjects. Dopamine receptor densities appear to be elevated in untreated schizophrenic patients,
57 whereas reduced receptor densities are reported in DAT patients
44—findings consistent with the differences in EPS observed in our patients.
Two small studies have directly compared
in vivo dopaminergic markers in DAT and MDD subjects. We found the prolactin response to neuroleptic challenge to be significantly more variable in 7 DAT patients when compared with 4 patients diagnosed with MDD with psychotic features.
58 Wolfe et al.
59 examined cerebrospinal fluid HVA in 9 patients with DAT, 4 patients with MDD, and 8 patients with idiopathic Parkinson's disease. Mean HVA concentrations were highest in the DAT group, followed in descending order by Parkinson's disease and MDD patients. Interpretation of these reports is limited, however, by the small number of patients studied. This is especially true since HVA is sensitive to both psychomotor retardation (associated with decreased HVA) and psychomotor agitation (associated with increased HVA) in patients with MDD.
60Interestingly, reduced dopaminergic function may also underlie the association between bradykinesia and depressive withdrawal in our sample. Several lines of evidence suggest that impaired dopaminergic neurotransmission may lead to the onset of depressive symptoms and psychomotor slowing.
61 In fact, in the report by Wolfe et al.,
59 when patients from all diagnostic groups were combined, those with low HVA concentrations demonstrated significantly more EPS, mental slowing, and depression than those with high HVA concentrations.
Several important limitations of the current study need to be addressed. Because of the cross-sectional nature of our study design, it is possible that some percentage of the observed EPS in our patients were actually the early manifestations of another neurologic illness that would become manifest if follow-up evaluations were conducted (for instance, subclinical Parkinson's disease in a patient with MDD). Conversely, it is possible that in some patients EPS occurred secondary to prior neuroleptic use and would dissipate on follow-up examination. Anecdotal reports have suggested that this form of persistent drug-induced parkinsonism may be common in the elderly.
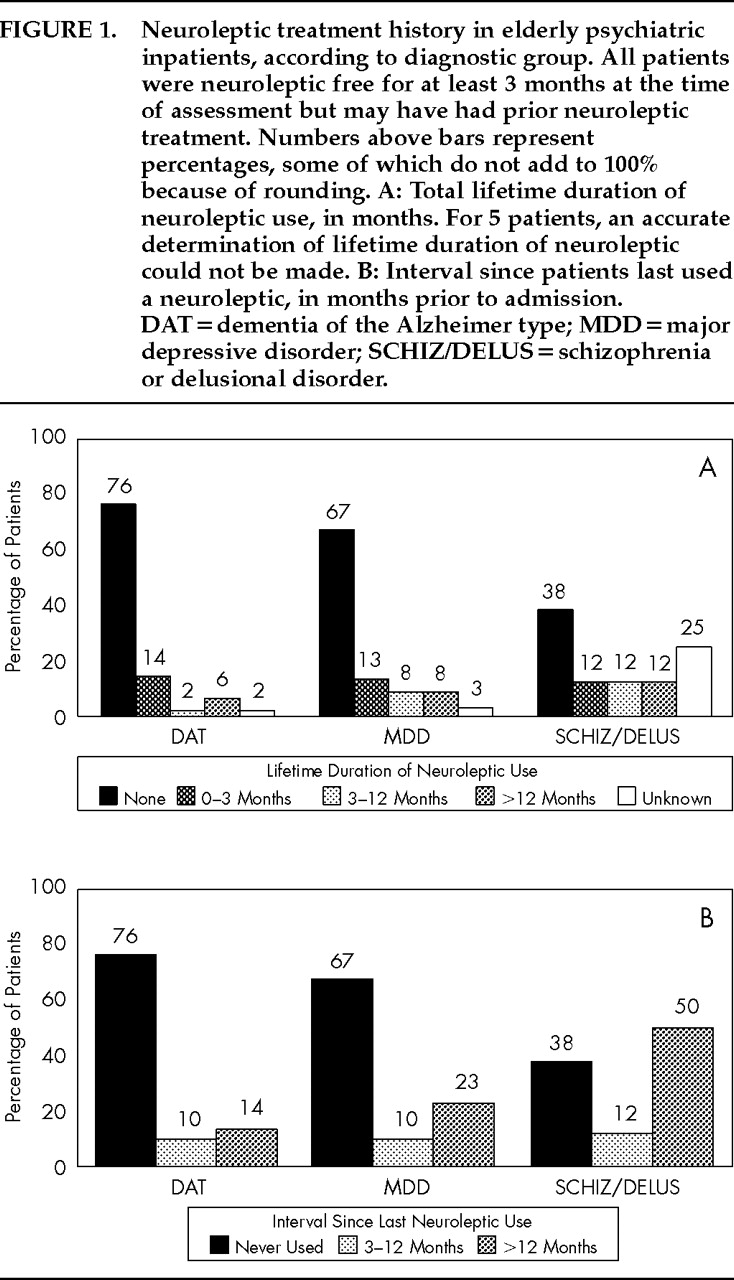
62 Our data (the first to address this issue of which we are aware) would argue against the occurrence of this syndrome. We found no association between EPS and either lifetime duration of neuroleptic use or interval since last neuroleptic use in our patients. Thus, if persistent drug-induced parkinsonism does occur, it would not appear to contribute substantially to the variance in EPS rates in the current study.
An unavoidable consequence of limiting our study to patients who were neuroleptic free for at least 3 months prior to admission was that the resultant SCHIZ/DELUS patients were limited in number and had neuroleptic treatment histories that were atypical for patients with these diagnoses. As discussed earlier, generalization from this group must be viewed with caution. It does not appear, however, that inclusion of this group in the analysis unduly influenced the results of the analyses conducted. Our principal findings—the independent association of rigidity with diagnostic group and the associations of bradykinesia with depressive withdrawal and gender—persisted when we repeated the multivariate analyses excluding the SCHIZ/DELUS patients. The magnitude of the significant associations between diagnostic group, depressive withdrawal, and EPS in our patients, however, should not be overstated. Diagnostic group accounts for only 6% of the variance in rigidity scores, while depressive withdrawal explains only 14% of the variance in bradykinesia. Similarly, none of the identified variables was significantly associated with the presence of tremor in our patients. The substantial unexplained variability in the determinants of EPS in elderly neuropsychiatric patients may not be surprising given the clinical and neurobiologic heterogeneity of these disorders. Further clarification of the determinants of EPS across subgroups of elderly neuropsychiatric patients will require investigative strategies that assess concurrently the effects of diagnosis, depressive slowing, and in vivo measures of dopaminergic activity and/or dopamine receptors.