First-rank symptoms, described by Kurt Schneider
1 as very specific to schizophrenia, are among the more florid manifestations of this disorder. They include the perception of voices speaking about the patients or to them (voices conversing or commenting); the belief that thoughts have been broadcast, stolen, or inserted (thought broadcasting, withdrawal, or insertion); and the belief that actions or thoughts are controlled by outside forces or other people (made actions, made thoughts). There has been much debate about the significance of Schneiderian symptoms in the diagnosis of schizophrenia. The original notion suggested by Schneider that they are pathognomonic for this disorder is inconsistent with the current construct of schizophrenia as defined by the recent editions of DSM. The specificity of Schneiderian symptoms for schizophrenia depends on the narrowness of their definition and is no longer considered to be as pathognomonic as originally proposed by Schneider.
2 In this report, we were interested in trying to better understand the neuroanatomic areas that may be involved in their production.
According to Schneider the primary common characteristic of these symptoms is that actions and personal states of the patients are not experienced as their own: patients suffer from a disorder of “selfness.”
1 Patients have lost the normal sensation of agency of their own thoughts and actions. In thought insertion, thought broadcasting, or thought withdrawal, personal thoughts and those of others are mixed, and during verbal hallucinations patients may hear speech that they do not recognize as their own and attribute the voices to someone else speaking around them. There is a loss of the intimate boundaries separating the self from others. This loss can express itself not only in patients feeling invaded by other people, but also in the inverse feeling of controlling others (which can express itself in megalomania, often a subjective sensation rather than a pathological intellectual interpretation). It involves problems in the attribution of thoughts and actions between patients and other people.
Schneiderian first-rank symptoms have been related to a defect in monitoring one's own actions and intentions
3,4 and to an impairment of a specific system allowing the attribution of the executed and seen actions to their respective authors.
5,6 This mechanism allowing the identification of the originator of an action should involve cerebral areas implicated in both execution and observation of actions. PET studies have shown that numerous brain regions are activated during such tasks but also that there is a cerebral pattern of activation common to the execution and the observation of a similar action.
7,8 This pattern includes the parietal lobe (Brodmann area [BA] 40), part of the supplementary motor area, the ventral premotor area, and the cerebellum.
We hypothesized that Schneiderian symptoms would be related to excessive activity in these regions linked to vivid mental imagery of action, leading to an impaired representation of the subject's and others' actions. A recent PET study concerning passivity phenomena in schizophrenia,
9 which are part of the Schneiderian symptoms, stressed the role of the right parietal cortex. To our knowledge, the present study is the first one to explore cerebral blood flow of schizophrenic patients in relation to the intensity of first-rank symptoms.
DISCUSSION
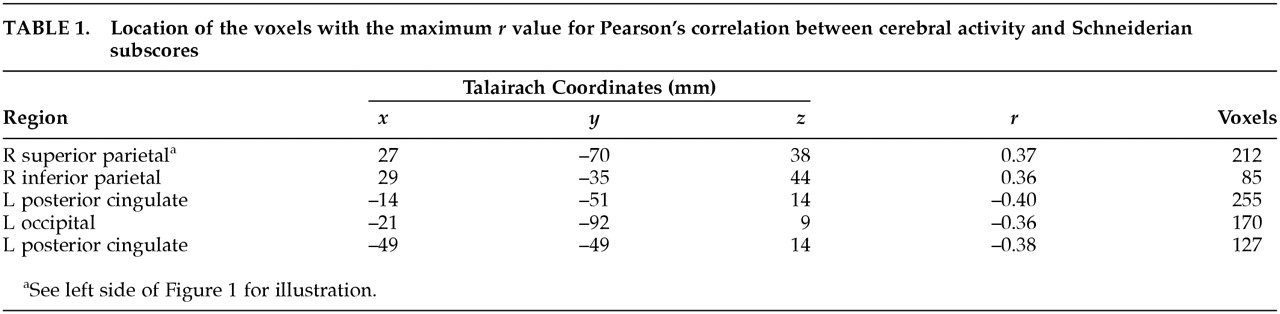
These results provide further support for a relationship between Schneiderian symptoms and increased blood flow of the right parietal cortex found in a previous study concerning a subset of the Schneiderian symptoms.
9 This parietal cortical association area is involved in perception of space and has been shown to be active during both execution and observation of grasping movements.
7,8 Our result strengthens the idea that Schneiderian symptoms are associated with an impairment in the mechanisms underlying the recognition of originators of actions. The left posterior cingulate region, in which rCBF had a negative correlation with Schneiderian symptoms, has been the subject of several studies. It projects to and receives afferents from the pulvinar, which is also connected with parietal and prefrontal cortex.
21 Its role in mental diseases remains to be clarified; however, an increase in activity in this region has been found in obsessive-compulsive disorder (OCD) just before the ritualizing phase.
22 We would suggest that this area may mediate the anxiety related to rituals. Increased activity in this area might also be related to the excessive self-control observed in OCD, whereas the inverse relation between posterior cingular activity and Schneiderian symptoms observed in the present study could be associated with a lack of control of the patients' own thoughts and acts. The negative correlation found between Schneiderian symptoms and the activity of area 18, which is a secondary visual area, is more difficult to explain. Decreased activity in this region could be related to abnormal mental imagery, rather than to overly vivid mental imagery.
23 The mental imagery of Schneiderian patients may be more related to physical activity, as indicated by the hyperactivation of area 40, than to visual activity, resulting in a decrease in occipital activity.
Other imaging studies concerning psychotic symptoms have been conducted. Several methodological differences between those and the present study make it difficult to directly compare their results and ours. For example, Liddle et al.
24 studied relationships between rCBF and schizophrenic symptoms in 30 patients. Although one of the three symptom factors was reality distortion, corresponding to delusions and hallucinations, they were studying broadly defined psychotic symptoms rather than more narrowly defined Schneiderian ones. Therefore, the pattern of rCBF that they found (positive correlations between reality distortion syndrome and left parahippocampal gyrus and left ventral striatum; negative correlation between that syndrome and right posterior cingulate) was related to psychotic symptoms in general.
Several studies have also examined verbal hallucinations. One of them,
25 using single-photon emission computed tomography (SPECT), scanned 12 patients when they were experiencing hallucinations and after that symptom has resolved. That study focused on regions of interest situated in frontal and temporal lobes and did not examine parietal lobes. Another study
26 analyzed regional brain metabolism on three slice levels in 12 patients who were hallucinating during the PET session. These two imaging studies were looking for the neural correlates of the hallucinating state and used restricted methods adapted to demonstrate the hypothesis of an abnormal inner language during auditory-verbal hallucinations. Other PET
27 or functional MRI
28 studies have examined involvement of auditory-language cortices in hallucinatory phenomena. Both of these studies focused on the brain events occurring when the patients experience hallucinations. The PET study
27 showed that patients with verbal hallucinations displayed maximal activity in the bilateral thalamus, left hippocampus, left parahippocampal gyrus, and right ventral striatum; these regions probably play a role in generating an internal representation of external world and in putting together mnemonic data, current perceptual data, and affective representations. The fMRI study
28 demonstrated the involvement of primary auditory areas in verbal hallucinatory phenomena. So, although structures implied in language production and in mnemonic associative functions are involved in hallucinatory production, Schneiderian symptoms considered as a whole are more related to abnormal body or action representation, as indicated by the involvement of right parietal cortex. This latter anomaly could lead to the loss of boundaries between self-generated and other-generated actions and thoughts underlying Schneiderian symptoms.
The present study treats Schneiderian symptoms as a group, linked by the concept of loss of autonomy or “selfness.” The results suggest that this group of symptoms is correlated with a brain dysfunction in areas implicated in action recognition (in particular, BA 40). Further imaging studies should confirm this correlation by using motor tasks
29 that produce a subjective sense of loss of autonomy. Such experiments will be helpful to understand relationships between cerebral functioning and psychotic symptoms.