Pathological laughing and crying (PLC) can be described as uncontrollable episodes of laughing or crying that are triggered by a stimulus that would not normally cause such an emotional response. In addition, PLC is a relatively frequent consequence of brain damage. It has been reported that the prevalence of PLC is approximately 10%–20% among patients with stroke,
1,2 40% among patients with Alzheimer’s disease (AD),
3 7%–10% among patients with multiple sclerosis,
4 and 19%–49% among patients with amyotrophic lateral sclerosis (ALS).
5,6Classic pathophysiological theories of PLC are based on the assumptions of serial processing and hierarchical control. According to these assumptions, PLC results from the release of cortical inhibition of upper brainstem centers that integrate the motor activation patterns involved in laughing and crying.
7 Thus, PLC is an essential part of the pseudobulbar palsy syndrome that is the consequence of bilateral lesions in corticobulbar pathways. However, PLC may also be seen in patients with unilateral lesions that do not involve motor or premotor areas. Ross and Rush
8 hypothesized that pathological affect could be observed in patients with lesions of the right inferior frontal lobe in association with a major depressive disorder. McCullagh et al.
9 studied ALS patients with and without PLC and implicated the prefrontal cortex in the pathophysiology of pathological affect. More recently, Parvizi et al.
10 suggested that the critical lesions eliciting PLC are located along fronto-ponto-cerebellar pathways.
There have been few studies focusing on PLC following traumatic brain injury (TBI). Zeilig et al.
11 reported the occurrence of PLC in 16 out of 301 patients with severe TBI consecutively admitted to a rehabilitation unit. Patients with PLC had a greater severity of brain injury and other neurological features compatible with pseudobulbar palsy.
The relationship between PLC, mood disorders, and anxiety disorders has not been consistently established. Previous studies focused on the relationship between PLC and major depression. Some studies reported that PLC was associated with the presence of major depression,
1,3,12 while others reported no association.
9,13,14 On the other hand, there have been few studies that have examined the association between PLC and the presence of anxiety disorders.
15Because PLC can be socially disabling, it may interfere with a patient’s rehabilitation process.
16 We have developed a quantitative scale to measure and quantify the severity of PLC, termed the Pathological Laughter and Crying Scale. This scale has high reliability and demonstrated validity and has been used to effectively rate PLC in patients with different neurological disorders.
2 There is also considerable evidence that PLC responds to treatment with antidepressants.
2,16,17 Using double blind methodology, previous studies have demonstrated that PLC responds to amitriptyline,
17 nortriptyline,
2 citalopram,
16 and sertraline.
18In this study, the clinical correlates and the clinical course of PLC following TBI were examined. We hypothesized that PLC was associated with damage to the prefrontal cortex and the presence of mood or anxiety disorders.
METHODS
Study Population
The study group consisted of 92 consecutive patients with closed head injury admitted to the University of Iowa Hospitals and Clinics (
n=58) and the Iowa Methodist Medical Center, Des Moines, Iowa (
n=34). Patients with penetrating head injuries or associated spinal cord injury were excluded. Patients with severe comprehension deficits that would preclude a thorough neuropsychiatric evaluation, defined as those who were unable to complete Part I of the Token Test,
19were also excluded from the study. Sixty-nine of the 92 patients with TBI (75.0%) were injured in a motor vehicle accident; 16 patients (17.4%) were injured by falls; three patients (3.3%) were injured by assaults; and the remainder (4.3%) were injured by other mechanisms (e.g., sports). All 92 patients gave written, informed consent for participation in this study. They were examined during their in-hospital stay and at 3, 6, and 12 months after injury.
Severity of Brain Injury
We used two measures of severity of TBI. The first measure was the 24-hour Glasgow Coma Scale score. According to this measure, Glasgow Coma Scale scores of 13–15 define mild head injury, 9–12 define moderate head injury, and 4–8 define severe head injury.
20 Patients with a Glasgow Coma Scale score in the 12–15 range but who underwent intracranial surgical procedures or presented with focal lesions greater than 15 cc total volume were considered to have moderate head injury.
21 The second measure of severity of TBI was based on the length of posttraumatic amnesia. The posttraumatic amnesia period was estimated retrospectively using a structured interview that has been shown to have a high correlation with prospective determinations of posttraumatic amnesia.
22In addition, the occurrence of medical complications such as hypoxia or arterial hypotension that might have contributed to secondary brain damage was assessed. Background, neurological, and neuroradiological data were registered and recorded using Traumatic Coma Data Bank forms.
23Definition and Assessment of PLC
A diagnosis of PLC was made according to the following criteria:
1.
The occurrence of frequent episodes of sudden, uncontrollable, emotional expression.
2.
Emotional responses were elicited by either nonspecific stimuli or, when elicited by appropriate stimuli, the intensity of the emotional response was out of proportion to the intensity of the stimulus.
3.
Episodes did not have a clear association with the prevailing mood state.
Severity of PLC was assessed by the Pathological Laughing and Crying Scale,
2 an interviewer-rated scale that quantifies aspects of pathological affect, including the duration of the episodes, relation to external events, degree of voluntary control, inappropriateness in relation to emotions, and degree of resultant distress. The scale consists of 16 items (eight assessing pathological laughing [PL] and eight assessing pathological crying [PC]) that are scored from 0 (rarely or not at all) to 3 (frequently).
Psychiatric Assessment
All patients were assessed by a psychiatrist using two semistructured interviews, a modified version of the Present State Examination
24 designed to elicit symptoms of mood and anxiety disorder and the Structured Clinical Interview for DSM IV Axis I Disorders.
25 Severity of depressive and anxiety symptoms was assessed using the 17-item Hamilton Depression Rating Scale
26 and the Hamilton Anxiety Rating Scale,
27 respectively. The Mini-Mental State Examination
28 was used as a global measure of cognitive functioning. Impairment in activities of daily living was assessed using the Functional Independence Measure.
29 Social functioning was quantitatively assessed using the Social Functioning Exam and Social Ties Checklist.
30 The reliability and validity of each of these instruments have been demonstrated previously in brain-injured populations.
31 Aggressive behavior was assessed using the Overt Aggression Scale.
32Neuroimaging
Computed tomography and occasionally magnetic resonance imaging (MRI) scans were obtained as part of the standard clinical evaluation in the emergency and neurosurgery departments of institutions involved in this study. The nature, extent, and location of traumatic lesions were classified according to the TCDB criteria, and registered using appropriate TCDB forms. Lesion locations were transferred to templates according to the methodology proposed by Damasio and Damasio.
33 A neurologist trained in the assessment of structural neuroimaging scans, who was blind to the results of the psychiatric examination, read each scan.
Data Analysis
Background characteristics, results of neuropsychiatric assessment, and mean Glasgow Coma Scale score were analyzed using Mann-Whitney U test (two-tailed) based on mean and standard deviations (SD). Frequency distributions of background characteristics and result of neurological assessment and the frequency of mood disorder, anxiety disorder, and lesion location were analyzed using chi-square tests (or Fisher’s exact test, two-tailed, if sample sizes were prohibitively small). Relationships between continuous variables were estimated by Pearson’s correlation coefficients.
RESULTS
Background Characteristics
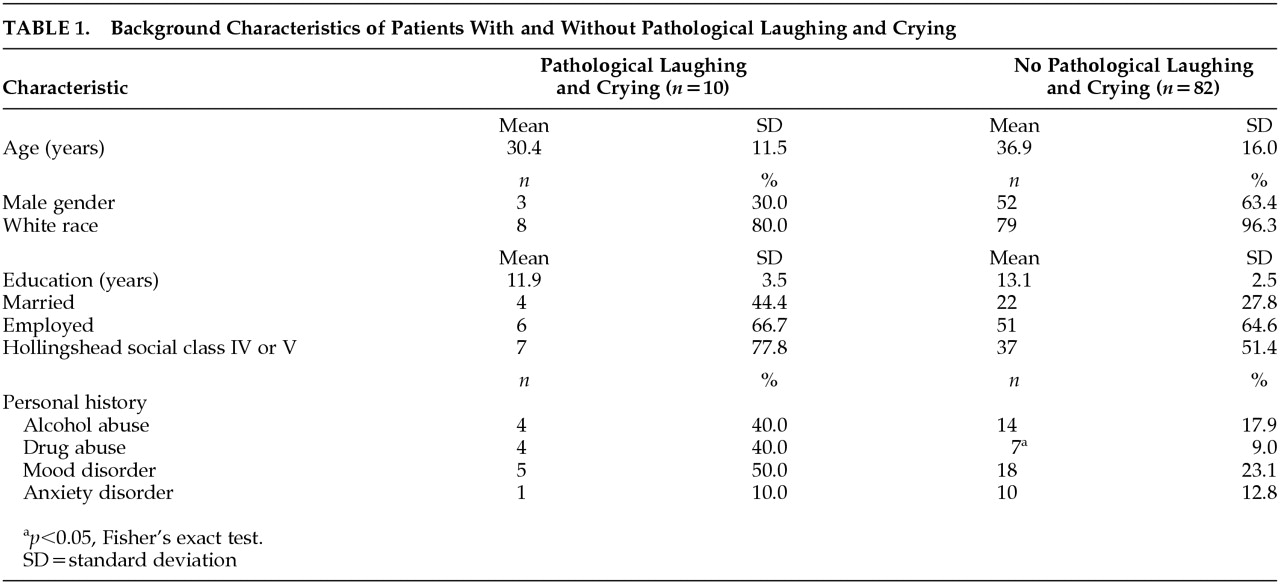
There were 10 (10.9%) out of 92 TBI patients who met our criteria for PLC. The prevalence of PLC was 3.3% at the initial evaluation, 7.1% at 3 months, 6.1% at 6 months, and 1.7% at 12 months. However, all patients developed PLC within 6 months after TBI. Seven patients demonstrated only episodes of PC, two patients demonstrated only episodes of PL, and the remaining patient demonstrated both episodes of crying and laughing. Demographic characteristics of patients with PLC and patients without PLC are shown in
Table 1. There were no significant differences between the two groups in terms of age, years of education, marriage or employment status, and Hollingshead socioeconomic class. The number of women and nonwhite patients was higher among the group of patients with PLC, compared to patients without PLC. However, these differences were not statistically significant (gender:
p=0.08, Fisher’s exact test; race:
p=0.09, Fisher’s exact test). There was a significant difference between the PLC and no-PLC groups in the frequency of personal history of drug abuse (
p=0.02, Fisher’s exact test). There were no significant differences between the PLC and no-PLC groups in the frequency of personal history of alcohol abuse, personal history of anxiety disorder, or in the frequency of personal history of mood disorders.
Clinical Correlates of PLC
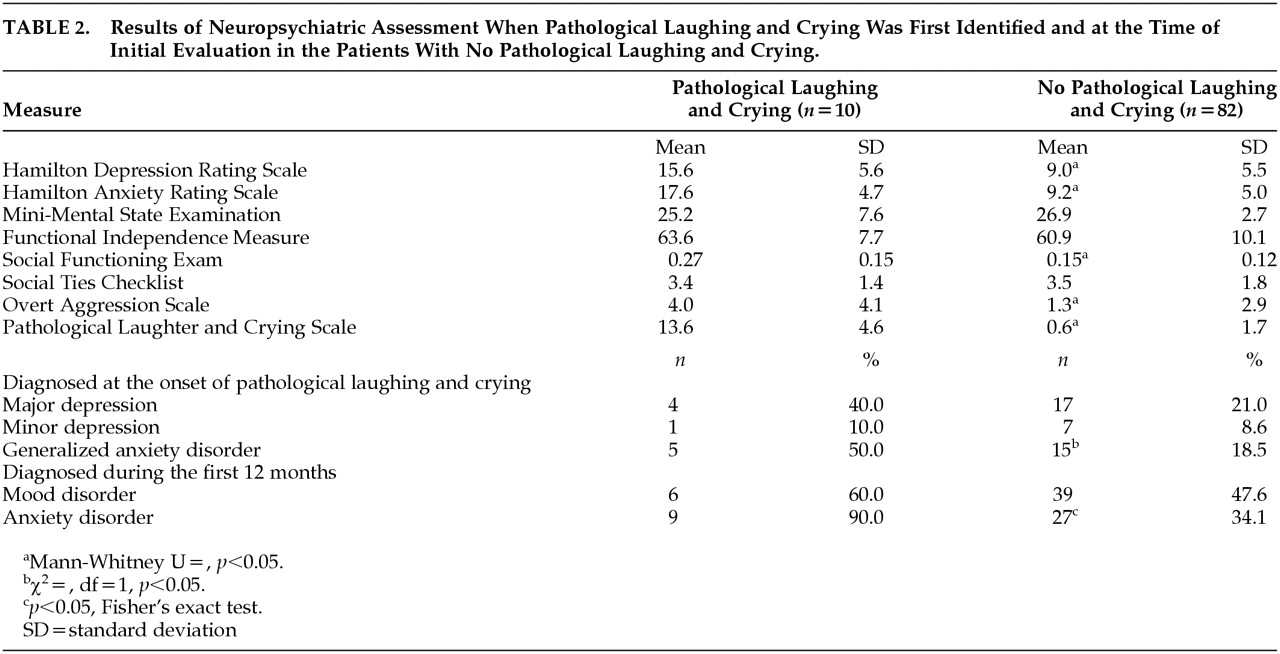
The results of neuropsychiatric assessment are shown in
Table 2. When compared with patients without PLC, patients experiencing PLC had significantly greater Hamilton depression scale scores (χ
2=10.48, df=1,
p=0.001), Hamilton anxiety scale scores (χ
2=15.71, df=1,
p<0.0001), and Overt Aggression Scale scores (χ
2=11.61, df=1,
p=0.0007). In addition, patients with PLC had significantly higher Social Functioning Exam scores (i.e., more impaired) than patients without PLC (χ
2=6.02, df=1,
p=0.01). The frequency of generalized anxiety disorder was significantly higher among patients with PLC (χ
2=5.15, df=1,
p=0.02). However, the frequency of major depression was not significantly different between the two groups. Patients with PLC were more likely to suffer an anxiety disorder (i.e., acute stress disorder, generalized anxiety disorder, panic disorder, or posttraumatic stress disorder) than patients without PLC during the first year after TBI (χ
2=11.57, df=1,
p=0.0007). However, the frequency of mood disorders (i.e., major depression, minor depression, or secondary mania) during the first year after TBI was not significantly different between the PLC and no-PLC groups.
Pathological Laughter and Crying Scale scores among patients with PLC were significantly correlated with Hamilton anxiety scale scores (r2=0.42, p=0.04) and Overt Aggression Scale scores (r2=0.47, p=0.03). The correlation between Pathological Laughter and Crying Scale scores and Hamilton depression scale scores was not statistically significant, however.
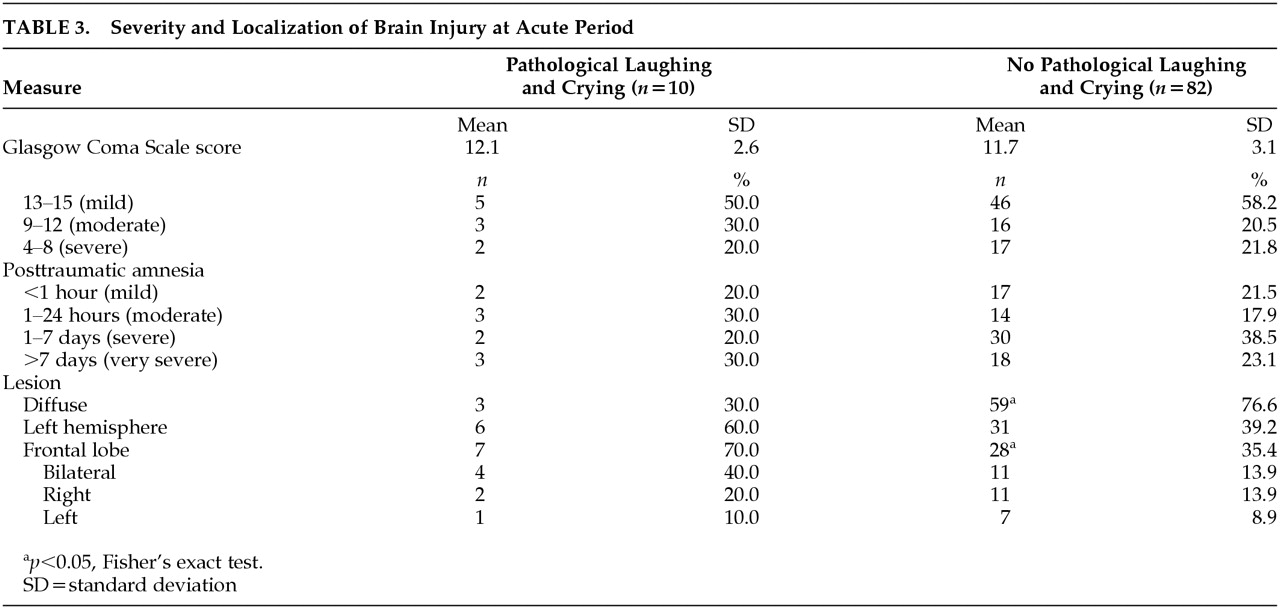
Severity and localization of brain injury at acute period are shown in
Table 3. Severity of brain injury measured by either Glasgow Coma Scale scores or the length of posttraumatic amnesia was not significantly different between patients with PLC and patients without PLC. In addition, the frequency of hypoxia and hypotension, the two most significant complications contributing to secondary brain damage, was not significantly different between the PLC group and no-PLC group (hypoxia: 0% versus 6.6%, hypotension: 12.5% versus 6.6%).
There were no significant between-group differences in the frequency of supratentorial or infratentorial lesions or the frequency of left or right hemispheric lesions. There was no significant difference between patients with PLC and patients without PLC in the frequency of temporal lobe lesion, parietal lobe lesion, or occipital lobe lesion. However, patients with PLC had a greater frequency of frontal lobe injury than patients without PLC (
p=0.04, Fisher’s exact test). In addition, there was significant difference between the PLC group and no-PLC group in the frequency of diffuse lesion according to the TCDB classification (i.e., PLC: type I–IV [diffuse lesion] 30.0%, type V–VI [mass lesion] 70.0%, no PLC: type I–IV 76.6%, type V–VI 23.4%) (
P=0.005, Fisher’s exact test). On MRI scans at 3 months after TBI, there was significant difference in the frequency of frontal lobe lesions between patients with and without PLC (
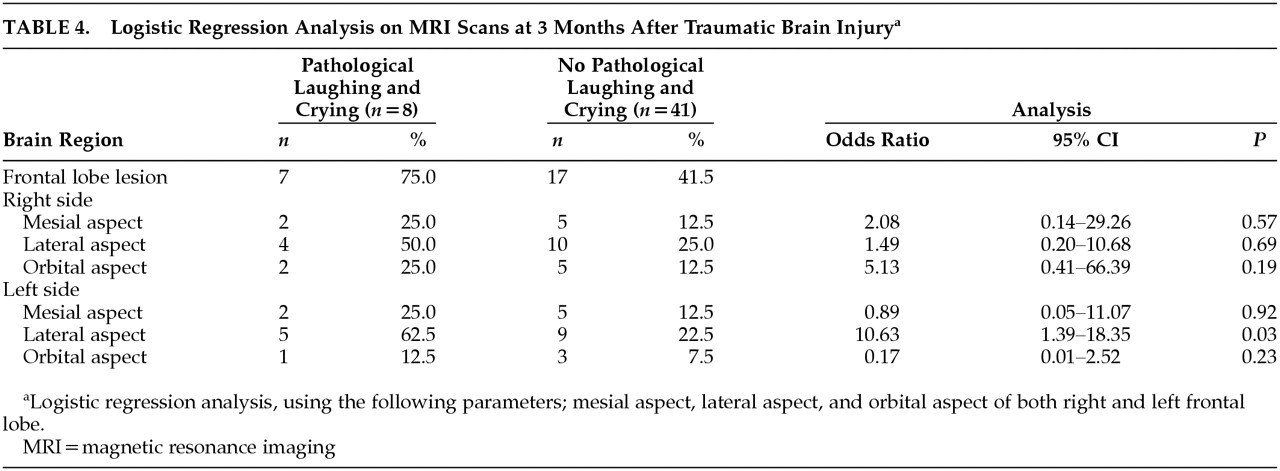
Figure 1) (
p=0.02, Fisher’s exact test). There were no significant between group differences in the frequency of lesions in other brain areas. Since the frequency of frontal lobe lesions was significantly different between patients with PLC and those without PLC, logistic regression analysis was used to determine which area of frontal lobe influenced on the presence of PLC. Logistic regression analysis showed that lateral aspect of left frontal lobe was associated with the presence of PLC (odds ratio=10.63, 95% confidence interval=1.39–18.35,
p=0.03) (
Table 4).
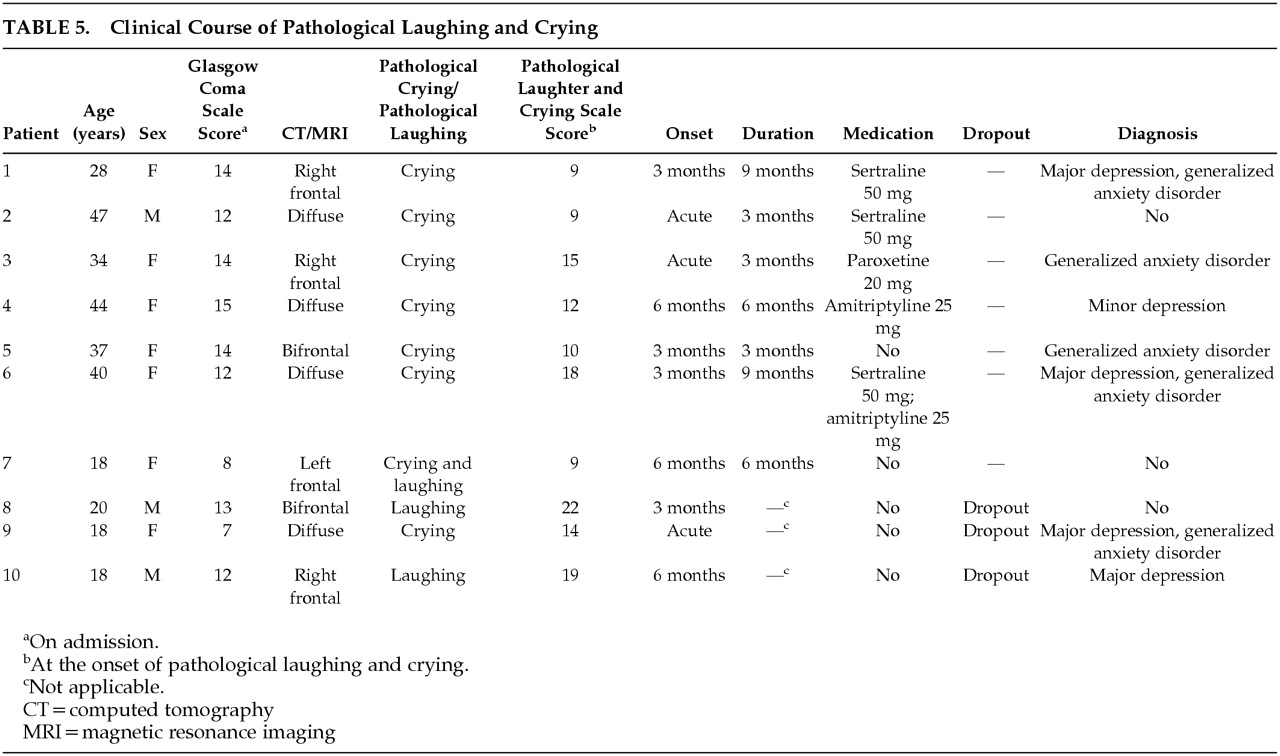
Clinical Course of PLC
Clinical characteristics of patients with PLC are shown in
Table 5. Of the 10 patients with PLC, three did not have follow-up evaluations after PLC was diagnosed and, consequently, their clinical course was not assessed. Five patients received antidepressants. Of these five patients, two received sertraline (50 mg/day); one received paroxetine (20 mg/day); one received amitriptyline (25 mg/day); and another received both amitriptyline and sertraline (25 mg/day and 50 mg/day). Also among these five patients, four showed complete remission of PLC within 3 months of starting antidepressants, while the remaining one showed a 50% reduction in PLACS scores at 3-month follow-up. Of the remainder who did not receive antidepressants, one recovered from PLC after 6 months and the other was free of PLC symptoms within 3 months of onset. Interestingly, this patient received gabapentin (400 mg t.i.d.) for treatment of neuropathic pain.
There were no significant differences between scores at onset and scores at the time of recovery from PLC in Hamilton depression scale scores (mean=14.7, SD=5.6, versus mean=15.4, SD=8.1), Hamilton anxiety scale scores (mean=15.9, SD=4.5, versus mean=14.9, SD=6.0), and Overt Aggression Scale scores (mean=3.3, SD=2.9, versus mean=2.0, SD=1.4). Thus, the resolution of PLC was independent of resolution of depression or anxiety symptoms. This may be related to the fact that the PLC patients sometimes responded to doses of antidepressants lower than those required for depression.
PL
The two patients who had episodes of PL but no episodes of PC were young males with moderate head injuries and right frontal lobe lesions. Compared to the other patients with PLC, patients with PL were more anxious (Hamilton anxiety scale scores were 19 and 22 versus mean=16.9, SD=5.0) and more aggressive (Overt Aggression Scale scores were 14 and 4 versus mean=2.8, SD=2.3). Unfortunately, they dropped out of our study, and their clinical course of PL could not be assessed.
DISCUSSION
In this study, a valid and reliable quantitative scale to assess PLC among patients with TBI was used. Findings revealed that 10 (10.9%) out of 92 patients showed PLC during the first year after TBI. Pathological laughing and crying was significantly associated with a previous history of drug abuse, the presence of aggressive behavior, the occurrence of anxiety disorders, and a significantly higher frequency of frontal lobe lesions, especially the lateral aspect of left frontal lobe.
Before discussing the implications of this study, it is necessary to acknowledge its methodological limitations. First, most of the subjects were young patients of Caucasian origin representing the population of Iowa. Thus, the findings may not pertain to other populations of TBI patients. Secondly, lesion analysis was based mostly on CT scans obtained during acute hospital stays. It is uncertain whether our findings would have been different if all the patients had been examined using more sensitive neuroimaging methods such as MRI.
Implications of this study are, however, thought-provoking. Contrary to previous reports, PLC was not associated with severity of TBI, although patients with PLC had evidence of greater prefrontal involvement, a finding that suggests a role for the prefrontal cortex in the etiology of this condition. Logistic regression analysis showed that the lateral aspect of left frontal lobe lesion was significantly associated with the presence of PLC. There is evidence of hemispheric specialization in processing the positive or negative valence of emotional stimuli.
35 Gelastic (laughing) seizures usually originate from the left hemisphere whereas crying seizures are mostly associated with right hemispheric foci.
35 In a recent study performed in healthy volunteers, Padberg et al.
36 reported that the frequency of laughing reactions elicited by a humorous video was increased after high frequency repetitive magnetic stimulation of the left dorsolateral prefrontal cortex. Conversely, PL and euphoria have been associated with right hemispheric lesions,
37 and PC has been observed in patients with lesions of the left hemisphere.
9 It is then conceivable that the prefrontal cortex integrates the complex sensory and limbic information that determine the emotional valence of a stimulus and modulate motor and autonomic responses involved in emotional expression. Abnormal prefrontal modulation of hypothalamic, pontine, and medullary centers that mediate these responses might produce PLC. Consistent with this view is the finding that patients with a previous history of drug abuse, who frequently show prefrontal pathology and frontal executive deficits,
38 are more vulnerable to develop PLC after TBI. Prefrontal dysfunction may also explain the observed association of PLC with aggressive behavior.
39Although patients with PLC had more mood symptoms, there was not a clear relationship between mood disorders and PLC. In fact, the association was stronger with the severity of anxiety symptoms and with the frequency of anxiety disorders, a finding, to our knowledge, that has not been previously described. Although an explanation of this association will be premature and highly speculative on the basis of the available data, we can hypothesize that abnormal activation of amygdala and, perhaps, other limbic and hypothalamic structures, contributes to the development of both PLC and anxiety symptoms. The fact that patients with stroke and PLC respond to treatment with a highly selective serotonin reuptake inhibitor (SSRI) such as citalopram
16 gives some support to the notion that serotonergic pathways are involved in the regulation of the neural circuits coordinating complex behavioral responses such as crying and laughing. However, the efficacy of SSRIs as treatment for PLC following TBI remains to be proved.
Finally, the fact that PLC might have a deleterious effect on social interaction and functioning underscores the need for a controlled treatment trial of antidepressants in patients with PLC following TBI.
ACKNOWLEDGMENTS
This study was supported in part by NIMH grants MH-40355, MH-52879, and MH-53592 and a grant from Nippon Medical School (Dr. Tateno).
The authors thank S. Rosazza and T. Kopel for gathering data and T. Kosier for assisting with data analysis.