Understanding how brain structures are functionally interrelated into networks is important to an understanding of psychiatric disorders and to interpreting the findings in functional neuroimaging studies. The recent emphasis on the neuroscience of emotions is of particular relevance. Current advances in the understanding of the neural circuitry of fear and how fear is modulated have widespread applications. Fear has a major influence upon memory, cognition, and behavior. Functional neuroimaging now allows researchers to probe the neural circuitry of both conscious and unconscious mental processes underlying fear and anxiety. It has also has been used to assess functional brain changes in response to different modes of therapeutic intervention. Insights gained from such studies may contribute to advances in treatment of anxiety disorders, including therapies with greater specificity for underlying brain abnormalities.
Fear is defined as “an unpleasant, often strong emotion caused by anticipation or awareness of danger”.
7 Feelings are conscious experiences that help in the identification of emotions.
8,9,10 When one feels afraid, one can identify the emotion of fear. “Not all feelings are emotions, but all (conscious) emotional experiences are feelings.”
8 The current understanding of fear circuitry in humans is based on animal research studies, imaging studies of human subjects with pertinent brain lesions, and, more recently, on human functional neuroimaging. Studies of both normal and pathological fear states (e.g., anxiety disorders) are relevant.
The Amygdala and Fear
Activation of the amygdala is central to generation of the fear response. The amygdala, in turn, activates areas of the brain important to measurable neurobehavioral correlates of fear, including the hypothalamus (release of the flight/fight hormones) and brainstem (freezing, startle).
8,9,11,12 The amygdala also causes widespread brain activation via its connections to the basal forebrain as well as cholinergic and noradrenergic centers in the brainstem.
9 Activation of the amygdala is required for acquisition of learned fear responses.
8–10,13–15 Learned fear states may be associated with increased excitability in the amygdala.
16,17 Animal research supports the concept that the amygdala and hippocampus act together to form long-term memories of affectively laden information and events.
18 The hippocampus is believed to link information about physical contexts with the emotional context provided by the amygdala.
14 Researchers believe that the amygdala strengthens memory consolidation during times of strong emotion.
9,12,15,17,18 Some types of fear-related memory may be stored in the amygdala.
Several interconnected areas are important for recall (retrieval) of fear-related memories including the amygdala, hippocampus, and anterior cingulate.
16,22 Although controversial, there is evidence that, once recalled (reactivated), a memory must undergo a new consolidation process (reconsolidation) in order to be maintained in long-term memory.
23–25 Thus while reactivated the memory may become vulnerable (labile) to modification or disruption. The reconsolidation process may involve the same areas as retrieval, although the processes appear to be different. The amygdala is also central to extinction of conditioned fear, whereby the fear response is weakened by multiple exposures to the conditioning context without the painful or frightening event.
15,22,26 Extinction does not remove the conditioned fear, but rather suppresses it by new learning.
22,26 The amygdala is activated during both acquisition and extinction of conditioned fear in humans.
22,27–29 Long-term storage of extinction-related memory to some extent depends upon modulation of the amygdala by medial prefrontal cortex (PFC).
15,17,22,29Current research indicates that external sensory information reaches the amygdala by two pathways. All sensory input is first relayed to the thalamus. Two divergent pathways emerge from the thalamus.
Direct pathways from the thalamus (thalamoamygdalar) can activate the amygdala very rapidly, on the basis of crude thalamic appraisals of sensory stimuli indicating potential danger (i.e., a long and thin object might be a snake). This reflexive activation of the amygdala has been referred to as “bottom-up” regulation of emotion.
30 The thalamoamygdalar route appears to operate at an unconscious level and can mediate fear conditioning independently of cortical input.
8,9,15Several lines of evidence from human studies support the existence of this pathway. The amygdala is activated by emotionally salient stimuli even when presented to the blind hemifield in a patient with an extensive left visual cortex lesion.
31,32 Various methods for presenting unconscious visual stimuli have been used in conjunction with functional imaging including backward masking or binocular rivalry to suppress conscious awareness in normal subjects.
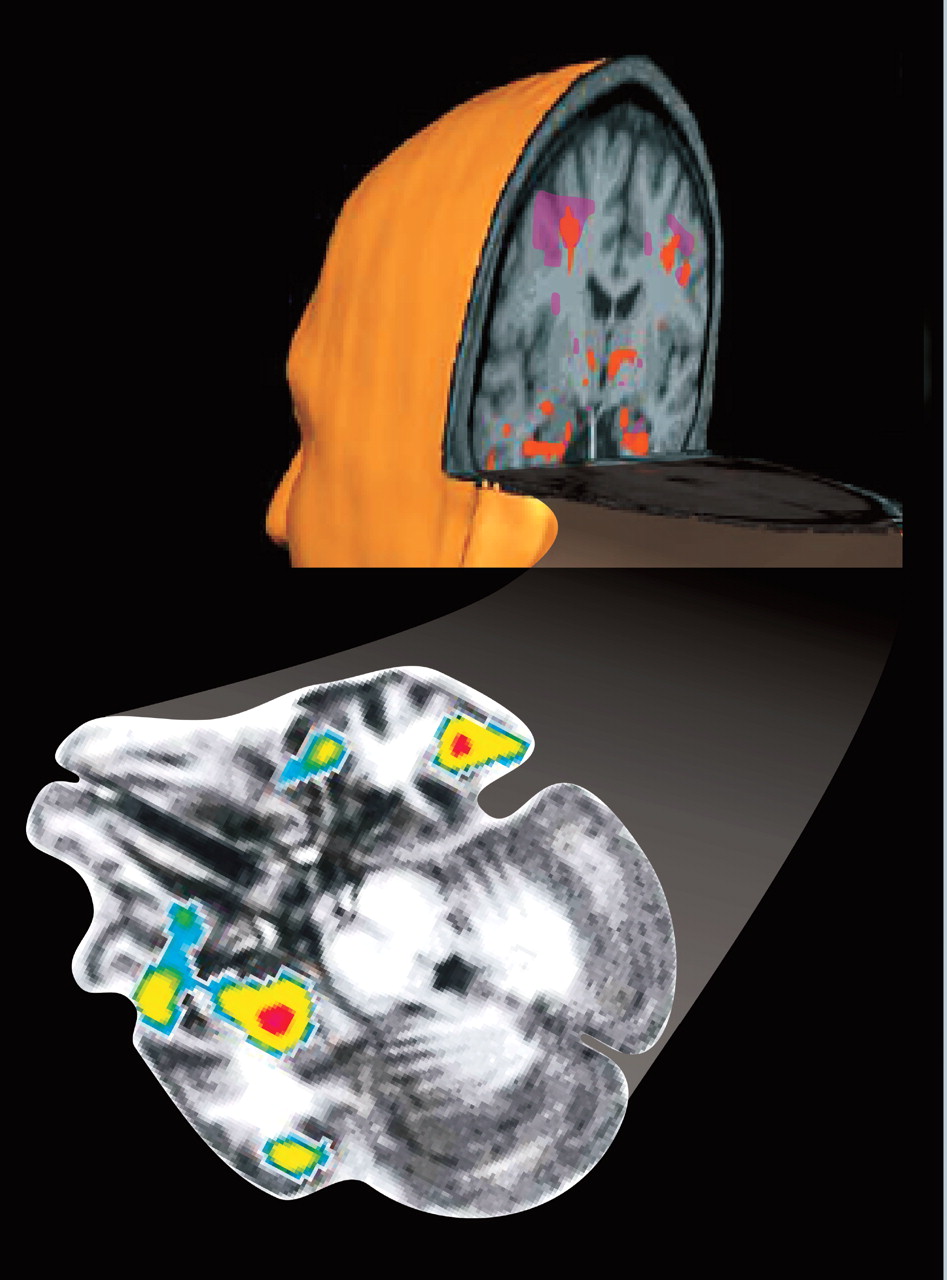
2,33,34 Most studies have reported activation of the amygdala during the suppressed condition to a range of emotionally salient images, supporting the existence of the direct pathway (
Figure 1). Conditioned responses can also be established with masked stimuli that activate the amygdala.
35 Studies in which attention to fearful or threatening stimuli was manipulated found that the amygdala was activated even when the stimuli were not attended to.
36,37 One study found that the amygdala responded to a broader range of stimuli during the “unattended” condition.
37 These studies support the concept that the amygdala has limited specificity when relying on information from the subcortical route (thalamoamygdalar pathways) and that this limited specificity reflects a trade-off between speed of processing and specificity.
Researchers believe that thalamoamygdalar pathways facilitate automatic, reflexive responses to a potentially aversive situation before it registers in conscious awareness.
8,38,39 The faster, imprecise, unconscious thalamoamygdalar pathway can activate the amygdala rapidly, which could mean the difference between life or death. However, such rapid amygdalar activation of a cascade of fear responses (e.g., release of epinephrine, norepinephrine, and cortisol) may overwhelm the capacity for conscious cognitive appraisals which occur via prefrontal cortical networks.
38Fear may enhance or interfere with attention, learning, and social judgements.
8,10,12,40 Damasio emphasizes the importance of intuition in decision making.
41 Studies in human subjects support the concept that unconscious mental processes influence conscious cognitions and feelings.
42,43 For example, studies employing subliminal emotional priming have demonstrated that test subjects liked or disliked a neutral stimulus (i.e., a Chinese ideogram or polygon) depending upon whether the stimulus was subliminally (unconsciously) primed by facial expressions of anger, fear, disgust, or happiness. Learned conscious and subliminal fear responses bias cognition and affective style.
13Alternatively, sensory input from the thalamus may reach the amygdala by
indirect pathways involving sensory cortices. The sensory cortices, in conjunction with other brain regions such as the hippocampus, parahippocampal, association, and prefrontal cortices, assign significance to sensory stimuli based upon context and prior experience.
9 Sensory information reaches the amygdala more slowly by this pathway, but conveys highly refined appraisals. These connections are reciprocal, allowing mutual regulatory influences.
8 Both the sensory cortices and amygdala are believed to relay information to PFC. Sensory information is not thought to enter conscious awareness unless processed in PFC networks concerned with conscious perception. The vast majority of sensory stimuli do not enter conscious awareness.
Modulation by Prefrontal Cortex
In neuropsychiatry, the PFC is commonly divided into three main divisions: the medial PFC (containing the anterior cingulate and paracingulate cortices), the orbital PFC, and the dorsolateral PFC. Medial PFC functions relevant to fear include attention to the emotional states of the self and others, guidance of response selection by emotional states, and suppression of fear-related behavioral responses as situations change.
15,19,22 Orbital PFC functions relevant to fear include modulation of behavioral and visceral responses associated with fear-related situations as situations change and modulation of emotional responses by correcting associations when they become inappropriate.
19,44 Dorsolateral functions relevant to fear are believed to include involvement in working memory, response preparation and response selection.
44The amygdala has reciprocal connections with orbital and medial PFC and is indirectly connected to the dorsolateral PFC. Orbital and medial PFC appear to exert a predominantly inhibitory influence upon the amygdala by activation of inhibitory interneurons (top-down modulation).
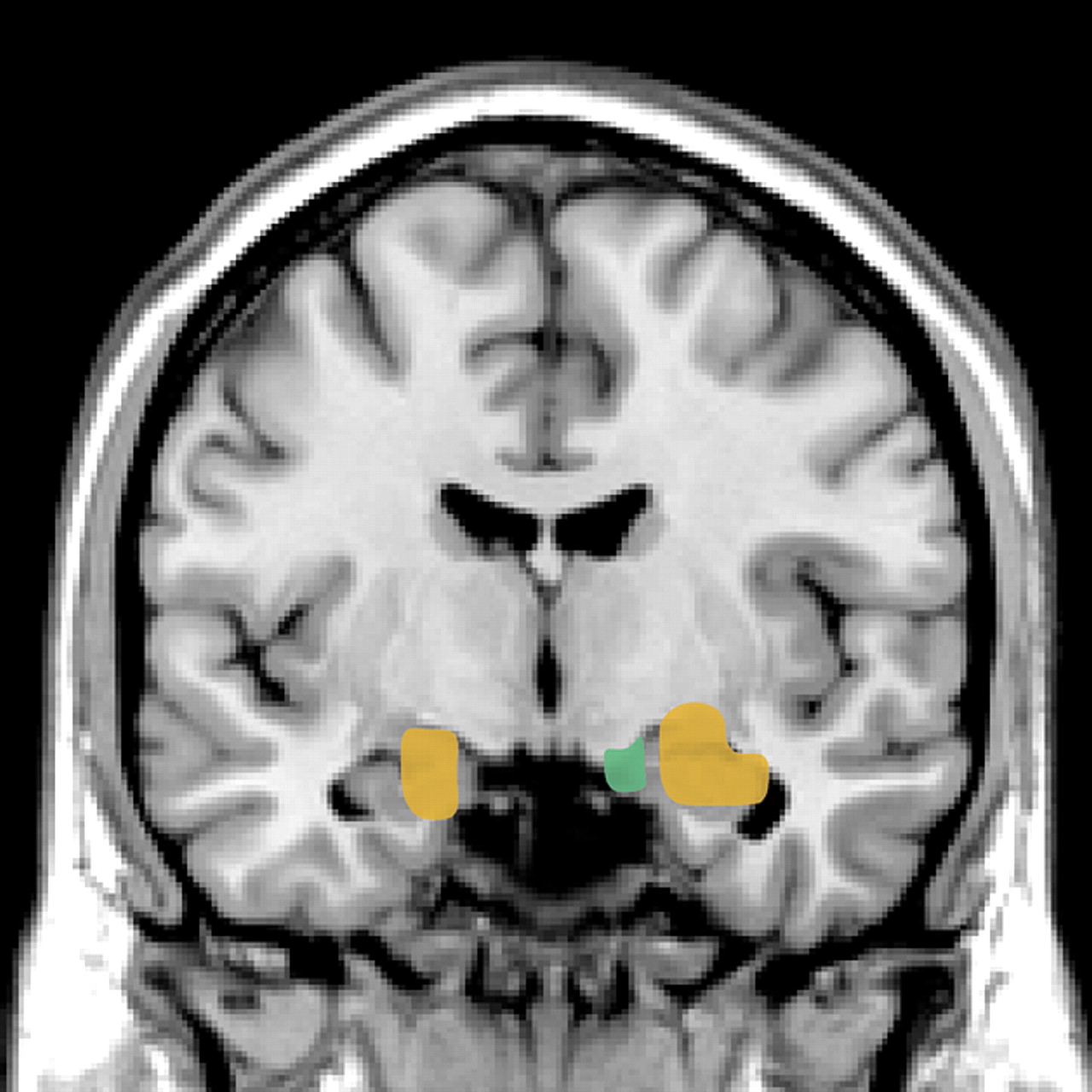
13,15,22 Several recent functional magnetic resonance imaging (fMRI) studies in normal subjects support top-down modulation of emotions by these areas of PFC. One group has compared patterns of brain activation while subjects perform tasks requiring conscious evaluation of emotionally arousing pictures versus simple matching tasks using the same pictures (
Figure 2).
4,5 When the 2 conditions were compared, stronger activation of the ventral PFC (BA 44/45 and 47) by the more complex evaluative tasks was associated with decreased activation of the amygdala. During the simple matching tasks, which evoked less activation of the ventral PFC, stronger activation of the amygdala was demonstrated. Autonomic reactivity as monitored by changes in skin conductance correlated with activation of the amygdala. A similar inverse correlation between activity in the amygdala and ventral PFC was also found in a study using subliminal priming with emotionally arousing stimuli.
3 In addition, both a gender-decision task and an emotion-identification task evoked less activation in the amygdala and more in ventral PFC than passive viewing of the same images.
45Modulation of the amygdala by the PFC may be one of the biological mechanisms which underlie the effectiveness of cognitive behavioral therapy (CBT) in anxiety disorders.
10,46,47 Similarities exist between extinction (in which a learned fear response is replaced or overlaid by new learning) and CBT.
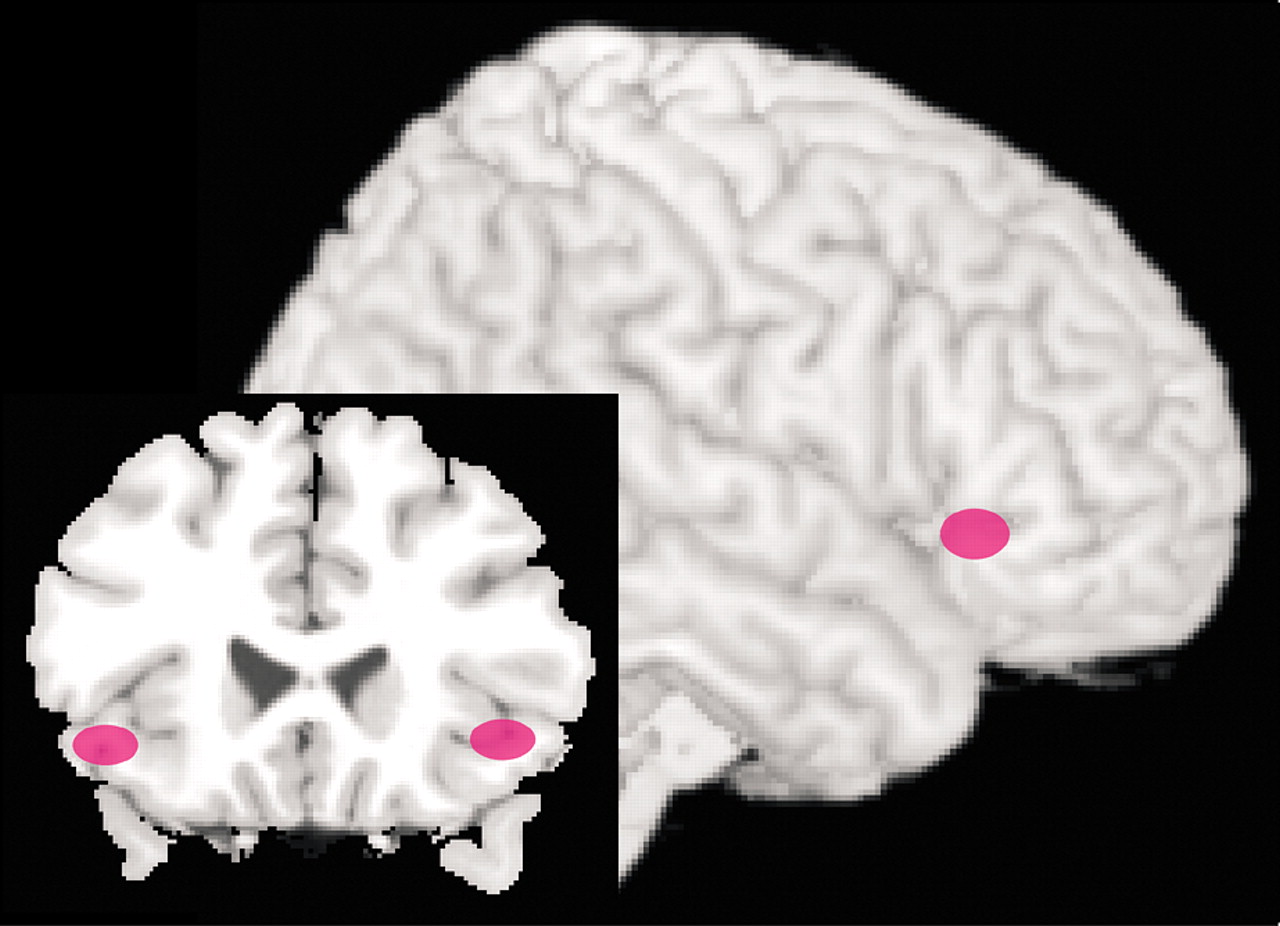
10,47,48 Two small studies have employed functional imaging to monitor the brain’s response to symptom provocation in patients with phobias (spider and social phobias) prior to and following therapeutic interventions (CBT was utilized in both studies and compared to treatment with citalopram in one).
6,49 In both studies patients that responded successfully to therapy exhibited decreased activity in limbic-related areas. In the study of patients with spider phobia, activation (measured by fMRI) in dorsolateral PFC (BA 10) and the parahippocampal gyrus during exposure to film excerpts depicting spiders was no longer found following successful completion of CBT.
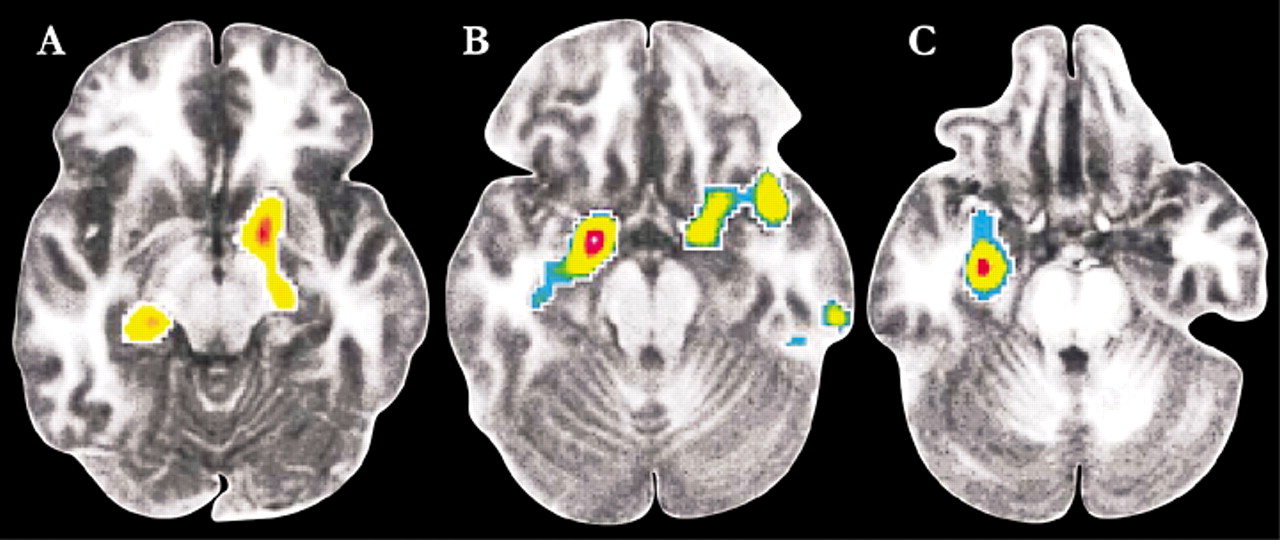
49 Similarly, in the study of patients with social phobia, those that responded well to either CBT or citalopram had decreased regional cerebral blood flow (measured by positron emission tomography) in the amygdala and hippocampus as well as periamygdaloid, parahippocampal, and rhinal cortices while performing a public speaking task (Figure 3).
6 In addition, the decrease in blood flow in the amygdala prior to and following treatment correlated with long-term clinical outcome. Both authors note that these results indicate that CBT is able to modify the abnormal neural functioning underlying anxiety disorders, perhaps by deconditioning or habituating contextual fear.
There is experimental evidence that extinction of fear can be facilitated by manipulation of neurotransmitters, specifically via the
N-methyl-d</-aspartate (NMDA) glutamate receptor.
50 In animal studies administration of D-4-amino-3-isoxazolidone (D-cycloserine, DCS) after extinction trials enhanced extinction. DCS is a partial agonist for the NMDA receptor, acting at the strychnine-insensitive glycine-recognition site. Efficacy of this approach for enhancing treatment of phobia was recently tested in a small double-blind placebo-controlled study.
51 Patients with acrophobia were randomly assigned to receive a high dose of DCS (500mg), a low dose (50mg) or placebo prior to 2 sessions of virtual reality therapy separated by 1-2 weeks. Presence of DCS did not affect level of fear exhibited during the first session, indicating no direct anxiolytic effect. During the second session and at follow up 1 week and 3 months later the patients that received DCS prior to therapy sessions exhibited significantly reduced fear of heights. These findings suggest that DCS may be a useful adjunct to therapeutic interventions for disorders in which fear-related learning is an important component.
Converging evidence supports the theory that genetic differences may influence development and/or expression of anxiety disorders. Increased reactivity of the amygdala has been demonstrated in individuals with a polymorphism in the promoter region of the serotonin transporter gene.
52,53 Presence of this polymorphism has been associated with susceptibility to fear conditioning as well as increased expression of anxiety and affective illness.
54–56 In patients with social phobia its presence is associated with both greater symptom severity and amygdala excitability.
53 It has been suggested that it may be associated with greater vulnerability to life stress.
57 These results are consistent with studies supporting a genetic component to all 3 phases of fear conditioning (acquisition, habituation, extinction).
58 It is likely that there is life-long interaction between biological factors (e.g., genetic) and environment in the establishment and re-modeling of networks involving fear and fear memory.
Functional imaging has demonstrated hyperexcitability of the amygdala in various anxiety disorders. Often an inverse relationship exists between activity in the amygdala and areas in PFC.
47 Recent studies illustrate this for social phobia and posttraumatic stress disorder (PTSD). Patients with social phobia exhibited increased regional cerebral blood flow (rCBF) (measured by PET) in the amygdala/periamygdaloid cortex during a public speaking task compared to controls.
59 At the same time, rCBF in orbital PFC decreased in the patients and increased in the controls. In another study, activation of the amygdala (measured by fMRI) did not differ between patient and control groups when viewing fearful or neutral faces (compared to happy faces).
40 However, the patients demonstrated significantly greater activation than controls in the amygdala and nearby cortex when viewing contemptuous or angry faces, indicating specificity related to their disorder. Larger than normal activations in the amygdala have also been measured in patients with social phobia during all phases of aversive conditioning (habituation, conditioning, extinction).
60 Patients with PTSD have also exhibited increased reactivity of the amygdala (measured by PET or fMRI).
1,61,62 Furthermore, measures of PTSD symptom severity were positively correlated with rCBF in the amygdala and inversely correlated with rCBF in medial PFC in one study.
62 Regional CBF in the 2 areas was inversely related.