Cataplexy is one of the most intriguing examples of how thought content can alter neurologic functioning. In a state of cataplexy, an intense emotional state triggers objective transient muscle weakness verified by areflexia.
1 The clinical manifestations are varied, ranging from involuntary eye closure and neck weakness to a subtle buckling of the knees to generalized muscle weakness that causes the patient to collapse.
2 Consciousness and awareness of the environment are preserved throughout the episode. Few clinical phenomena better illustrate the ability of an emotional experience to cause neurochemical alterations that result in observable behavioral signs.
Known primarily as a symptom of the sleep disorder narcolepsy, cataplexy, in extremely rare cases, has been associated with other disorders, which include Nieman-Pick disease type C, Norrie’s disease, mid-brain tumors, and familial isolated cataplexy.
3 When associated with excessive daytime sleepiness, with or without any other narcolepsy symptoms, cataplexy is considered pathognomonic of narcolepsy. Because of this close association, the pathologic features of narcolepsy provide valuable information about the factors conferring vulnerability to cataplexy. Recently, narcolepsy with cataplexy was associated with a deficiency of the neuropeptide hypocretin 1 (also known as orexin A), which is produced by a small number of cell bodies located only in the lateral hypothalamus.
4 The hypocretin neurons project widely throughout the central nervous system, including the spinal cord. Hypocretin 1 deficiency appears to play a permissive role, allowing certain emotional states to cause rapid shifts in downstream neurotransmitters, which results in cataplexy.
The decreased muscle tone observed in cataplexy is similar to the absent electromyographic tone documented during rapid eye movement (REM) sleep. Therefore, cataplexy is believed to represent dissociated REM phenomena intruding into wakefulness after an emotional trigger. The proposed mechanism implicates postsynaptic inhibition of spinal alpha motoneurons by activated cells, including cholinoceptive ones in the midbrain, pontine, and medullary regions.
5,6The specific aim of this study was to investigate which emotional experiences triggered cataplexy, observe the manifestations of the episode and determine whether a brief survey that would aid in identifying cataplexy could be developed. The negative and positive predictive value of the abbreviated survey was also measured.
RESULTS
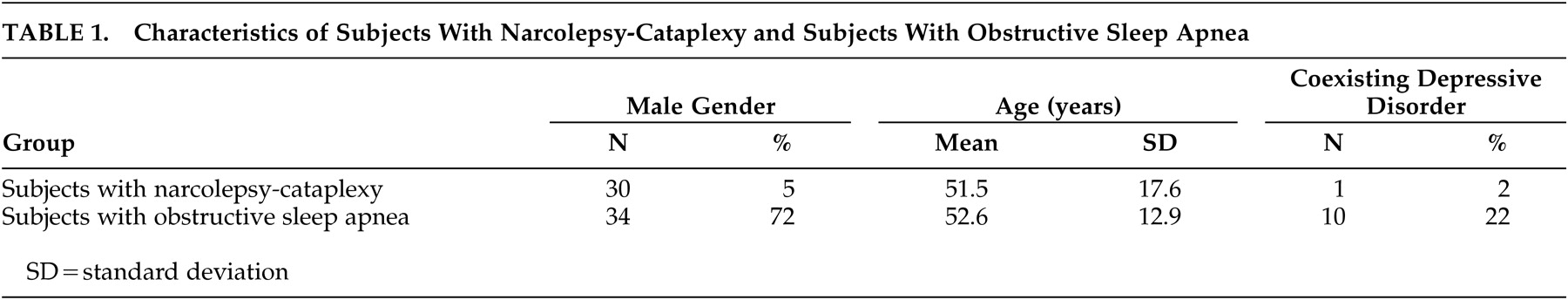
Table 1 provides demographic and clinical data concerning the narcolepsy-cataplexy subjects and comparison subjects. Few of the narcolepsy-cataplexy subjects had current or past psychiatric disorders. In both groups, the most common coexisting psychiatric disorder was depression. The most common comorbid medical disorder was hypertension in four (7.2%) of the narcolepsy-cataplexy subjects and 14 (30%) of the comparison subjects. Eleven (20%) of the narcolepsy-cataplexy patients had obstructive sleep apnea. The majority of patients with narcolepsy-cataplexy were receiving treatment from one or more pharmacological classes at the time of the study: psychostimulants, 35 (64%); modafinil, seven (13%); antidepressants to suppress cataplexy, 21 (38%); and/or hypnotics to improve nocturnal sleep, five (9%).
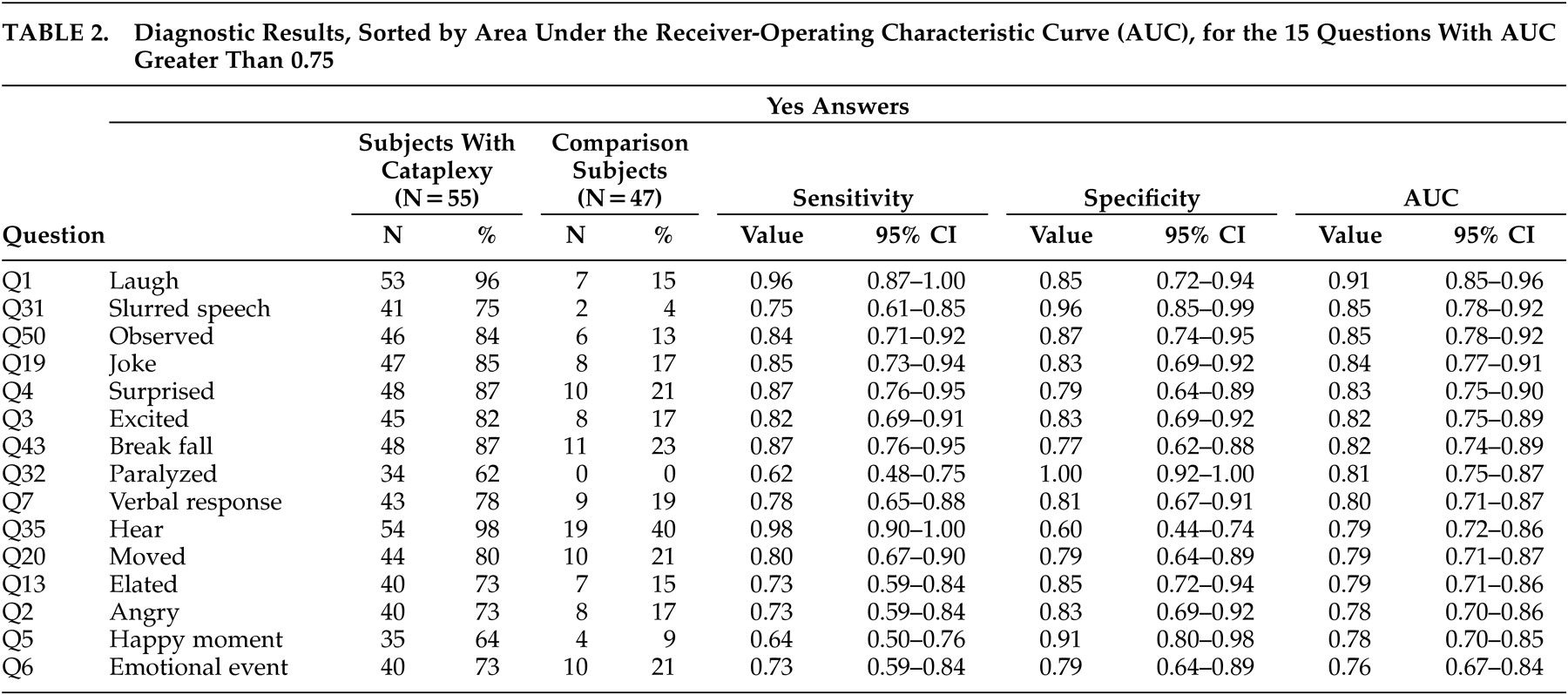
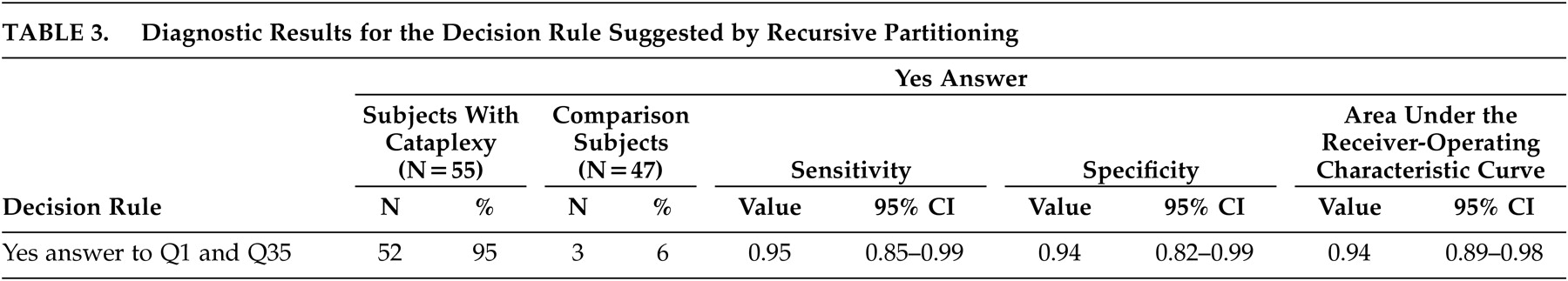
Table 2 shows diagnostic results for the 15 questions with AUC greater than 0.75. Question 1, regarding laughing results, has the highest AUC, at 0.91 (95% CI: 0.85, 0.96). Note that the AUC confidence interval for Question 1 overlaps with the confidence intervals for 13 other questions. Recursive partitioning results suggested splitting first, based on Question 1, and splitting second, based on Question 35 (ability to hear during the spell), providing that the answer to question 1 was yes. The resulting decision rule is to classify patients as having cataplexy if the answers to both questions 1 and 35 are yes and to classify patients as not having cataplexy if the answer to either Question 1 or 35 is no.
Table 3 shows diagnostic results for this decision rule. Sensitivity (0.95) and specificity (0.94) are nearly equal, and the AUC (0.94) is higher than for any single question. Additionally, the AUC confidence interval overlaps with the confidence intervals for seven of the single questions.
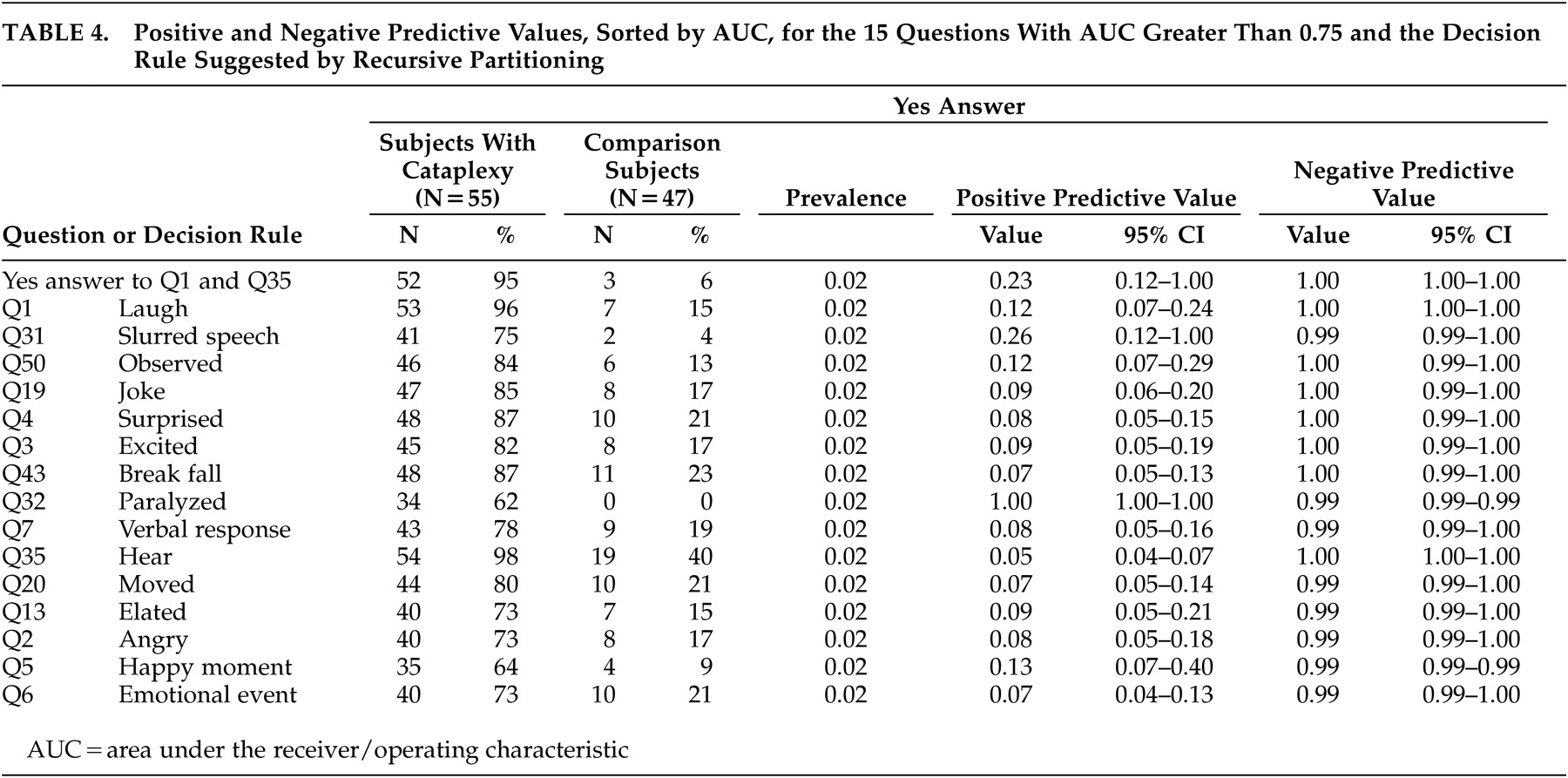
Table 4 shows positive and negative predictive value for the 15 questions with AUC greater than 0.75 and the decision rule suggested by recursive partitioning. Negative predictive value is near 1.00 for all presented questions. The positive predictive value is higher for questions 32 or 31 than for the decision rule suggested by recursive partitioning. Moreover, the positive predictive value for Question 32 is equal to 1.00 due to lack of false positives.
DISCUSSION
This study confirmed the results of Anic-Labat,
8 which suggest that laughter (Question 1) stands out as the emotional trigger most likely to provoke cataplexy. Patients with narcolepsy-cataplexy identified laughter as a more consistent cause of cataplexy than other closely related positive emotional states such as hearing a joke (Question 19), feeling excited (Question 3), feeling elated (Question 13), remembering a happy moment (Question 5), or experiencing an (unspecified) emotional event (Question 6). Laughter, a behavior unique to primates, is understudied. Studies on laughter have found that it consists of a process with repeated fluctuations of muscle activity and vocalizations spaced on average 210 ms apart.
12 A recent report published in abstract form, revealed that the frontal lobe regions are activated on functional magnetic resonance imaging scans when subjects are amused but instructed not to laugh.
13 The frontal lobes also determine social behavior and emotional judgment. The effect of laughter on neuronal firing in normal humans or narcoleptic subjects is unknown. Evidently, the hypocretin deficiency renders the subject vulnerable to laughter, causing abrupt transitions in neurotransmitters, putatively with increases in cholinergic activity, and reductions of norepinephrine and serotonin functioning.
14 The mechanism by which laughter could result in the activation of cells in the midbrain, pons and medulla and cause inhibition of spinal motoneurons is currently unknown. The factors that determine the cessation of a cataplectic episode are also unknown but are suspected to be related to increases in norepinephrine and serotonin relative to acetylcholine. Antidepressant medications, particularly those that increase norepinephrine levels and suppress REM sleep, reduce the frequency of cataplectic episodes.
15The analysis of the questionnaire data examined the emotional triggers and the features of a cataplexy episode.
Table 2 lists two characteristics of the episodes that are important for clinicians to recognize: slurred speech (Question 31) and being able to hear (Question 35). Subjects with narcolepsy-cataplexy also described having enough warning to break their fall (Question 43) and feeling paralyzed (Question 32). The differential diagnosis of spells is extensive. Slurred speech (related to muscle weakness), in the context of being able to hear auditory stimuli, helps distinguish narcolepsy-cataplexy from syncope, seizures, and other spells involving loss of consciousness. These features may also help distinguish narcolepsy-cataplexy from pseudocataplexy related to psychologic issues.
16 Many subjects reported that others had witnessed a cataplexy episode (Question 50), a condition that raises suspicion for pseudoseizures that typically occur in public rather than solitary settings. However, the social nature of laughter would explain such an observation for narcolepsy-cataplexy.
This study has several possible limitations, including a relatively small sample size. Subjects may have had difficulty recalling events that may have occurred in the past, and no test-retest data are available. Another concern is that there is the remote chance that subjects in the obstructive sleep apnea comparison group had co-existing narcolepsy-cataplexy. The dramatically clear findings, when the responses of the two groups are compared, make this unlikely, however. Nonetheless, narcoleptic-cataplexy patients have a higher rate of obstructive sleep apnea than the general population. The procedure for diagnosing narcolepsy in patients with obstructive sleep apnea involves performing additional diagnostic testing if sleepiness persists after treatment. The comparison group in this study is more clearly defined than the one used by Anic-Labat
8, which consisted of an undifferentiated sample of patients with varied sleep complaints, excluding clear-cut narcolepsy-cataplexy. Future studies could utilize a comparison group consisting of insomnia patients or the general population, although the utility of this questionnaire to distinguish patients with narcolepsy-cataplexy is expected to prevail.
Another potential limitation to this study is that participants may not have correctly understood the items on the questionnaire. Querying subjects about “muscle weakness” following a strong emotion is difficult, particularly for comparison subjects. Cataplexy is a condition so unusual that respondents may attempt to identify an incident that matches the description. The comparison subjects may have endorsed the experience inappropriately if they confuse narcolepsy-cataplexy with a syncopal spell, seizure, or other paroxysmal event. In our opinion, patients with narcolepsy-cataplexy are likely to recognize immediately the unique state they have experienced and correctly describe the events. The resulting bias is that the questionnaire may have resulted in false-positives in noncataplectic patients rather than false-positives or -negatives for the patients with first-hand experience of narcolepsy-cataplexy. Again, the robust findings reported here mitigate this situation.
In the absence of a reliable human cataplexy test, survey research about cataplexy takes on a special importance. This study confirms the findings of Anic-Labat that a questionnaire succeeds in identifying cataplexy in narcolepsy-cataplexy patients compared with a comparison group. This tool becomes particularly valuable if narcolepsy with cataplexy represents a disease with a different pathogenesis than the less common and more controversial condition of monosymptomatic narcolepsy (excessive daytime sleepiness without cataplexy). The hypocretin 1 data support this new segregation of narcolepsy into two distinct disorders since hypocretin deficiency has been strongly associated only with narcolepsy-cataplexy.
17,18 If testing of cerebrospinal fluid levels of hypocretin 1 becomes a widely accepted diagnostic test for narcolepsy, then it would be prudent to verify the presence of narcolepsy-cataplexy with a valid and reliable survey before exposing the patient to the risks of a lumbar puncture.
The receiver-operating curve results indicate that this lengthy survey may be distilled from 51 questions to 2 questions (experience muscle weakness when laughing and ability to hear during the spell), with high sensitivity and specificity. In the future, an abbreviated version should be carefully evaluated with narcolepsy-cataplexy patients and comparison subjects. These selected questions could then be included into brief screening tools for use with sleep, community, or psychiatric patients. Once narcalepsy-cataplexy is recognized as a possibility, physicians can refer patients for appropriate laboratory testing. Narcolepsy-cataplexy remains an underdiagnosed disease worldwide, largely because of a lack of convenient and reliable screening. Comprehensive surveys remain useful for research purposes for patients with identified narcolepsy-cataplexy since collecting data about the disease facilitates research into this fascinating mind-body interface. Optimistically, functional neuroimaging studies that provide additional information about the specific neural pathways and neurochemical interactions involved in the disease will be performed in the future.