The brain is now recognized as having its own innate immune system
6 and sharing a variety of bioactive molecules with the peripheral immune system. Morphological “delegates” of the immune system, the microglial cells, are present in the brain, and, respectively, the peripheral lymphoid organs are innervated.
7 These findings have led to the view that the brain and the immune system speak a common biochemical language.
8 The exchange of signals between immune and neural cells may lead to dysregulation of cytokine release.
9Alzheimer’s disease is a neurodegenerative disorder resulting in major cognitive decline. The main pathological hallmarks are the numerous senile plaques, the neurofibrillary tangles, and the cerebrovascular amyloidosis in various regions of the brain.
10 Aβ amyloid, a protein derived from beta-site proteolytic processing of the amyloid precursor protein, is the major component of senile plaques.
11 Inflammatory responses have been implicated in the pathogenesis of Alzheimer’s disease and deposition of Aβ amyloid. Lesions of Alzheimer’s disease are associated with low-grade but sustained inflammatory responses.
12 Activated microglia have been demonstrated in pathological lesions in several neurological diseases, including Alzheimer’s disease.
13,
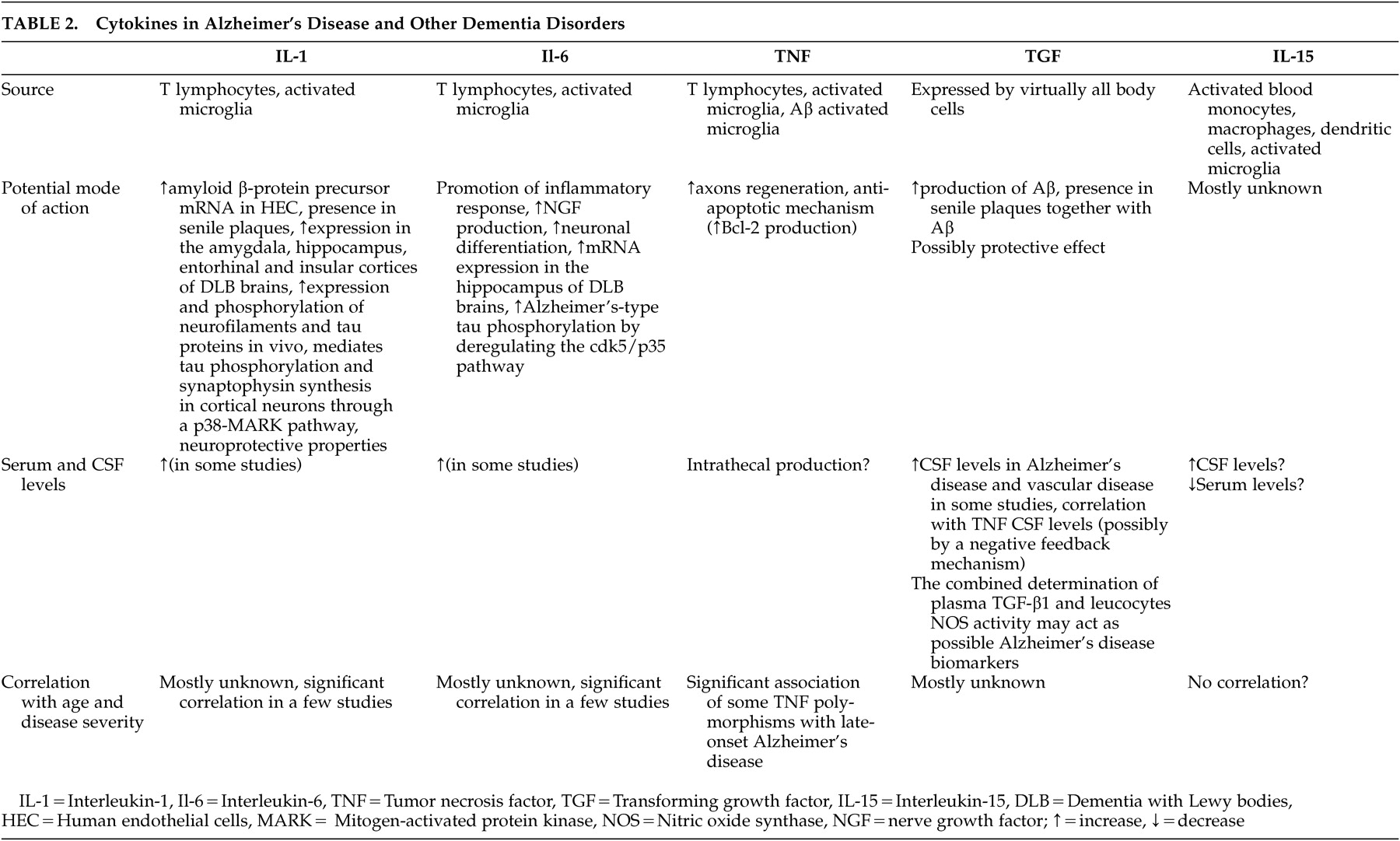
14 The presence of the acute phase proteins α1-antichymotrypsin, α2 macroglobulin (α2-M), C-reactive protein, and proinflammatory cytokines interleukin-6 and interleukin-1 in senile plaques has also been demonstrated.
15 –
18 Adhesion molecules are presumably involved in brain tissue lesions of patients with Alzheimer’s disease.
10,
19 Cells exposed to proinflammatory cytokines have elevated levels of amyloid precursor protein, which may result in increased production of Aβ and further plaque formation.
20To clarify whether IL-15 is involved in the possible inflammatory reactions of Alzheimer’s disease and other dementias as a marker of proinflammatory activation, we measured serum IL-15 levels in patients with Alzheimer’s disease and vascular dementia compared with healthy subjects. We decided to include patients with vascular dementia because Alzheimer’s disease and vascular dementia are the most common senile dementias. Cerebrovascular pathology increases the risk of developing Alzheimer’s disease and is involved in mechanisms of its progression.
21 Vascular risk factors interact with apolipoprotein (APO) E ε4 to increase the risk of Alzheimer’s disease beyond that of APOE ε4 alone.
22,
23 The vascular component of Alzheimer’s disease is important and potentially treatable.
24 Therefore, treatment of vascular risk factors may prevent Alzheimer’s disease or delay its progression.
Previous studies
25,
26 have demonstrated a link between cholinergic activation and amyloid precursor protein metabolism. The potentiation of central cholinergic activity has been proposed as a therapeutic approach for improving the cognitive function in Alzheimer’s disease patients.
27,
28 Results of recent studies suggest that treatment with acetylcholinesterase inhibitors in Alzheimer’s disease patients modulates the expression and production of proinflammatory and anti-inflammatory cytokines.
9,
29,
30 To assess the contribution of achetylcholinesterase inhibitors in the modulation of cytokine network presumably involved in Alzheimer’s disease neurodegeneration, we measured serum IL-15 levels before and 4 months after treatment with a regimen of donepezil (10 mg/day).
METHOD
A total of 50 subjects were enrolled in the study, divided in 3 groups: 1) the Alzheimer’s disease group comprised 20 patients with probable Alzheimer’s disease, diagnosed according to the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria;
31 2) the vascular dementia group comprised 15 patients diagnosed according to the National Institute of Neurological Disorders and Stroke and the Association Internazionale pour la Recherche et l’Enseignement en Neurosciences (NINDS-AIREN) and Hachinski Ischemic Scale (HIS);
32,
33 3) the comparison group comprised 15 healthy individuals. Patients with evidence of systemic inflammation on clinical examination or serum biochemical tests (increased number of white blood cells, elevated C-reactive protein, elevated erythrocyte sedimentation rate) were excluded. Patients with use of nonsteroidal anti-inflammatory drugs (NSAIDs), acetylsalicylic acid, steroids, or statins during the last 2 months before enrollment were also excluded from the study.
Sample Collection and IL-15 Assay
Serum concentrations of circulating IL-15 were measured by immunoassay using commercially available enzyme-linked immunosorbent assay (ELISA) kits (R and D Systems, Minneapolis, Minn.). Serum samples diluted 1:3 were added in duplicate to microtiter wells and assayed according to routine procedures. Optical densities were determined by means of a micro-ELISA reader (L.P 400, Pasteur Diagnostics). Values were calculated by comparison with a standard curve that was generated with IL-15 standards. The limit of detection was 2 pg/ml.
Statistical Analysis
All variables were tested for normality and homogeneity of variances by the Shapiro-Wilk’s and Leven’s tests, respectively. We observed significant deviation from normality and heterogeneity of variances in IL-15. However, logarithmic transformation restored the above violations to some degree and permitted the use of analysis of covariance (ANCOVA), with diagnostic group and sex as factors and age as covariates, followed by Newman-Keuls post-hoc tests. The results were confirmed by a Kruskal-Wallis test of original data, followed by Dunn’s post-hoc tests. The effects of age, duration of the disease, dementia severity, and sex on serum IL-15 levels were also tested separately in each group by the Spearman correlation coefficient and Mann-Whitney U test. We used repeated-measures ANOVA to compare pre- and posttreatment levels of IL-15 in Alzheimer’s disease log-transformation and confirmed them by Wilcoxon matched-pairs test.
DISCUSSION
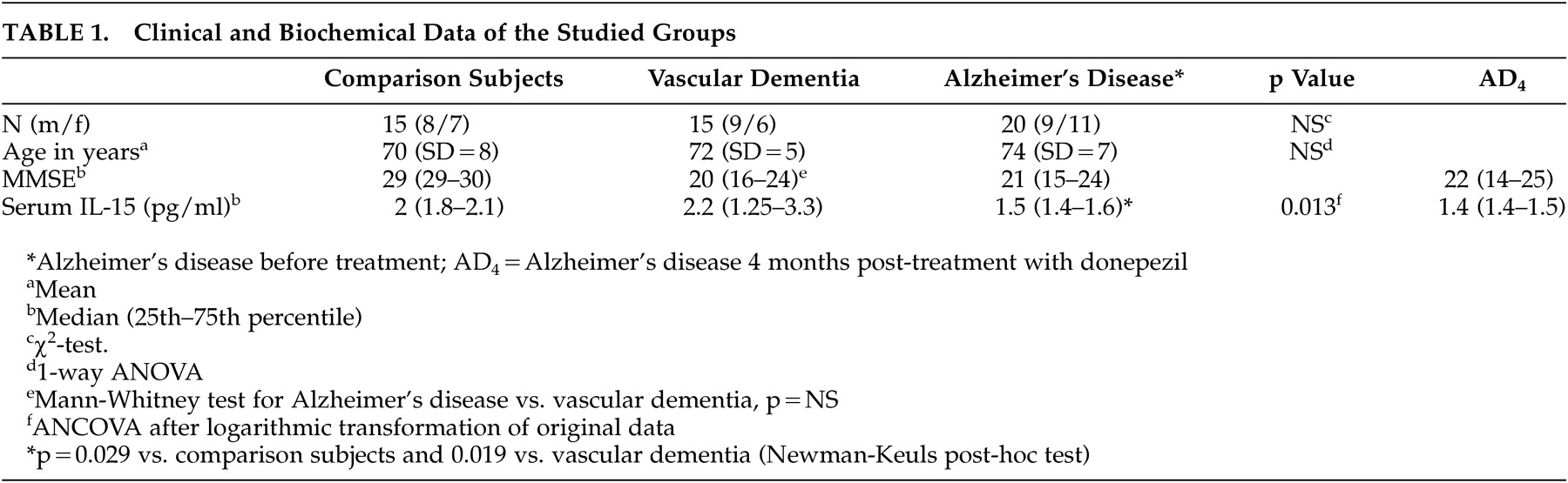
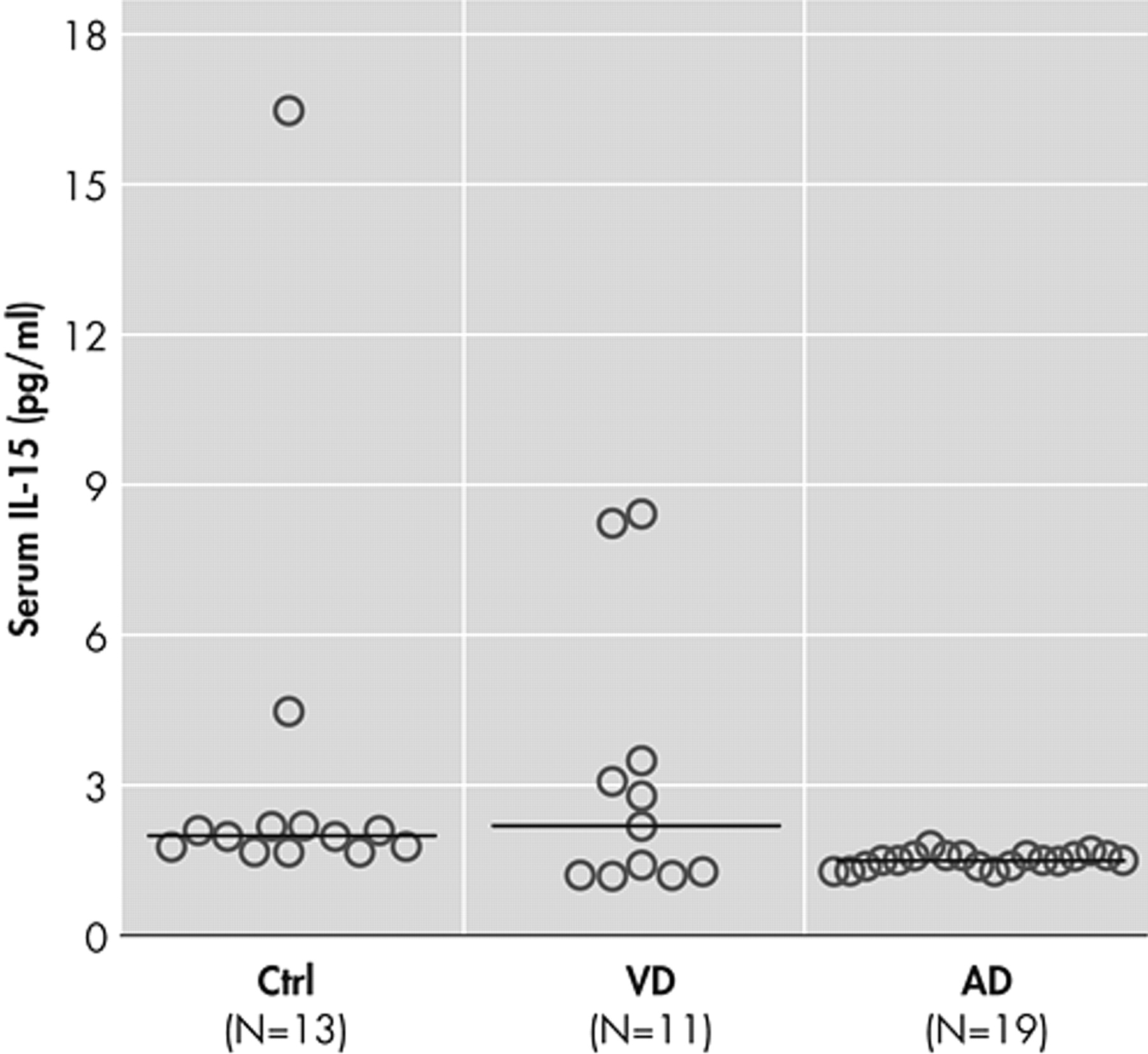
In the present study, we investigated serum levels of IL-15 as potential markers of inflammatory reactions in patients with Alzheimer’s disease and vascular dementia. Our data contradict the proinflammatory hypothesis since patients with Alzheimer’s disease had reduced serum IL-15 concentrations compared with the healthy group (
Figure 1 ). A significant decrease of interleukin-1beta and interleukin-6 secretion has been observed in severely demented patients
34 after lipopolysaccharide-stimulation of blood cells of Alzheimer’s disease patients with different disease severity. In another study, it has been shown that TNF-α release from blood cells is significantly decreased in demented patients compared to healthy subjects.
35 Our findings in the serum of Alzheimer’s disease patients come to support these latter results but seem to contrast with the conclusions of other recent reports which suggest an increased production
36 or increased circulation of cytokines.
37 Circulating cytokines have a short half-life, act mainly in an autocrine or paracrine fashion, may reach high concentrations at sites of release but much lower concentrations after dilution in blood, or they may bind to molecules that do not permit their detection by immunological methods.
38 All of these possibilities may factor into the contradictory results of several studies. An immune hyporesponsiveness to human Aβ has been found in amyloid precursor protein transgenic mice.
39 Patients with chronic exposure of the immune system to Aβ could have reduced immune responses, and this may explain why severely demented patients have reduced cytokine release. The low IL-15 serum levels in our patients with Alzheimer’s disease may indicate the existence of an effective mechanism that dampens the inflammatory response. The hyperactivity of the hypothalamus-pituitary-adrenal axis in Alzheimer’s disease patients leading to cortisol hypersecretion
40 may contribute to this phenomenon since glucocorticoids are potent inhibitors of cytokine production.
41 However, no correlation between serum IL-15 levels and duration or severity of the disease has been found in our study. This seems in contrast with other studies suggesting that the levels of other cytokines like TNF-α or IL-6 may be correlated with disease severity.
38 It is possible that IL-15 is not a marker of disease duration or severity.
Cytokines found in plasma or serum may be produced by blood cells, endothelium, or may originate from the brain. Activated by Aβ amyloid accumulation, blood cells may cross the blood-brain barrier and contribute to Alzheimer’s disease degeneration.
42,
43 In contrast, inflammatory molecules produced in the brains of demented patients may result in an inflammatory response in the periphery by humoral, neuroendocrine, and sympathetic connections that have been clearly demonstrated.
44 –
46 To clarify the issue of the source of circulating IL-15, its expression and production by activated blood mononuclear cells should be assessed.
There is evidence that inflammatory mechanisms are implicated in patients with cerebrovascular disease. The onset of cerebral ischemia may trigger a cascade of proinflammatory molecular and cellular events.
47 Some biochemical inflammatory markers, such as Erythrocyte Sedimentation Rate, levels of C-reactive protein, IL-6, S100β, intercellular adhesion molecule-1 (ICAM-1) and matrix metalloproteinase-9 (MMP-9), may be associated with early signs of ischemia in the neuroimage, with early and late clinical outcome, with the volume of the infarct, with hemorrhagic transformation, and with the efficacy of thrombolytic therapy.
48 –
50 In patients with cerebral infarct, an increase was found in the levels of acute phase proteins like C-reactive protein, fibrinogen, α-1 antitrypsin, and acidic α-1 glycoprotein, suggesting that ischemic necrosis is associated with inflammatory reactions.
50 Ischemia and hypoxia in vascular dementia patients could lead to the degeneration of nerve fibers.
51,
52 The activation of glial cells for clearance of damaged tissue could lead to the production of cytokines like IL-1, IL-2, IL-6 and TNF-α. In our study serum IL-15 levels were not different in patients with vascular dementia compared to healthy subjects. By visual inspection of the data (
Figure 1 ), it seems that some patients with vascular dementia have low IL-15 levels in the range of those with Alzheimer’s disease and the rest have higher levels in the range of healthy subjects. It is possible that patients with low levels of IL-15 have mixed dementia since it is known that many patients with the diagnosis of either Alzheimer’s disease or vascular dementia based on clinical examination have been proved to suffer from mixed dementia. Available data in the literature concerning cytokines and vascular dementia are contradictory and do not allow definite conclusions.
38,
53,
54 It is obvious that more comprehensive clinical trials need to be carried out before firm conclusions can be drawn concerning discrimination of dementias based on proinflammatory cytokines serum profiles.
Acetylcholine is synthesized by lymphocytes and released from activated T-cells into the vicinity of acetylcholine receptors of target cells, interacting with the receptors before hydrolysis by AchE.
55 –
58 Recent investigations have provided evidence that peripheral blood mononuclear cells possess most of the essential components needed to constitute a cholinergic system.
59 Acetylcholine synthesized and released from peripheral blood mononuclear cells may act as an immunomodulator.
56,
60 AChEI in our Alzheimer’s disease patients had not changed significantly serum IL-15 concentrations. IL-15 may act in a different way or may not be produced at the same time regarding other proinflammatory cytokines like IL-1β or TNF-α. In the present study, serum IL-15 levels were measured 4 months after onset of AChEI therapy instead of after 1 month in other recent studies.
9,
30 It is possible that the change of serum concentrations of IL-15 and other cytokines after treatment with AchEI, suggesting a remodelling of cytokine network, is not a lasting process. Specific mechanisms blocking the inflammatory response after an initial stimulation may be involved. It is difficult to clarify the exact role of so many inflammatory molecules, including cytokines, in the inflammatory responses that may be involved in the pathogenesis of dementias. Therefore, the failure of AChEI to influence IL-15 production does not exclude the possible correlation between the cholinergic system and immune responses in Alzheimer’s disease patients.
Achetylcholinesterase inhibitors appear to increase acetylcholine bioavailability but it is not completely known how they exert their probable neuroprotective action. Recent reports suggest that accumulation of Aβ amyloid and proinflammatory cytokines during aging may generate in the brain a “neurotrophin resistance” state that places the brain at risk for cognitive decline and dementia.
61To our knowledge, this is the first study in which serum IL-15 concentrations are assessed in patients with dementia disorders, such as Alzheimer’s disease and vascular dementia. It has some weaknesses, as do all studies that measure cytokine levels and relate them to the pathogenesis of dementia. Increases or decreases in cytokine levels, if they occur, may be transient or may differ according to the stage of illness. Hence, it would be important in future experiments to examine levels serially to know whether levels are stable or show periods of great increase or decrease. The half-life of cytokines may also differ. Therefore, similar increases or decreases in the production of cytokines at one moment may be associated with significantly different levels measured at a particular later time. Hence, single measures may not indicate levels over time. Immune markers can be synthesized in the CNS, in peripheral blood, or can be made by cells in one location that move to the other. Ultimately, the important levels for neurodegenerative disorders are in the CNS. In one recent study,
62 patients with Alzheimer’s disease and frontotemporal dementia had significantly higher CSF IL-15 levels compared with patients suffering from noninflammatory neurological diseases.
62 This may suggest an intrathecal IL-15 production. A possible explanation for these results (high CSF IL-15 levels in one study and low serum IL-15 levels in the other) is that we used patients with other noninflammatory neurological diseases in one study and healthy subjects as comparators in the other. Hence, it is not possible to exclude a potential impact of other neurological diseases on intrathecal cytokine production. It would be important to measure simultaneously CSF and serum IL-15 levels in the same patients in order to assess whether they are related.
Future studies of a greater number of patients that assess circulating cytokines in serum and CSF and/or cytokine levels in various regions of CNS by immunohistochemical methods must be carried out in order to investigate the peripheral or glial source of IL-15 and other cytokines in patients with dementias and to clarify the hypothesis of immune activation as part of the pathogenetic mechanisms in these disorders.