W e recently proposed that right insular dysfunction due to preclinical Alzheimer’s disease or other neuropathology may mediate fall and syncope related morbidity and mortality in nondemented elderly.
1 This is predicated on our observation of a specific association between visuospatial cognitive measures and mortality in nondemented elderly retirees.
2 Similar tests are known to predict mortality in Alzheimer’s disease,
3 while right hemisphere lesions are known to increase the risk of arrhythmic morbidity and mortality in stroke,
4 head injury,
5 and epilepsy.
6 Strokes that affect constructional tasks often involve the right insula as well.
7 Right insular stroke
8 or epileptic foci
9 often present with asystole. We hypothesize that preclinical Alzheimer’s disease, which is known to affect the insular cortex at an early Braak stage,
10 is responsible for both our observed mortality in nondemented elderly retirees and “age-related” autonomic dysfunction in septuagenarians and octogenarians.
11 Our preliminary studies in press elsewhere
12 suggest that insular neurofibrillary tangle counts moderate the associations between both age, QT interval, and death. Forty percent of nondemented octogenarians have Braak stages consistent with insular involvement, and may be at risk.
13 In this study, we examine the association between right insular cerebral blood flow (rCBF) by perfusion MRI (pMRI) and measures of cardiovascular autonomic control, in nondemented retirees (Clinical Dementia Rating Scale score ≤1.0),
14 carefully screened to be free of comorbid cardiovascular disease.
METHODS
A convenience sample of 29 community dwelling elderly volunteers was tested with measures of cognition, autonomic function, and pMRI. Participants were recruited from noninstitutionalized levels of care in a for-profit San Antonio comprehensive care retirement community. After giving informed consent, participants were screened for diabetes, clinical ischemic heart and cerebrovascular disease by self-report, ECG, two dimensional echocardiography (2D-ECHO), and T2 weighted MRI. Resting insular rCBF was determined by pMRI. Forty-two subjects were recruited. However, 13 were eventually excluded due to abnormal ECG (n=5), a technically inadequate scan (n=2), intolerance of the pMRI (n=5), or on the basis of a Clinical Dementia Rating Scale score greater than 0.5 (n=1).
Neuroimaging
pMRI experiments were performed on a 1.9 T Elscint Prestige MRI scanner (Elscint Ltd., Haifa, Israel) at the Research Imaging Center of the University of Texas Health Science Center at San Antonio. Baseline images were used to rule out occult focal right hemisphere/insular cortical lesions as the cause of any constructional dyspraxia/autonomic dyscontrol. Any cases found to have either mass lesions or focal cortical infarctions/hemorrhagic lesions were excluded.
Cerebral Blood Flow
Absolute cerebral blood flow (CBF) was measured by dynamic contrast MRI using singular value decomposition with an adaptive threshold developed in our laboratory.
15 A single-shot multiple-slice spin Echo Planar Imaging pulse sequence was used with the following parameters: TR/TE/ =1500 msec/80 msec/90 (0), slice thickness =5 mm, and an in-plane spatial resolution of 3.3 mm by 3.3 mm. For each study, multiple-slices (120 images per slice) were obtained during a total scan time of 3 minutes. All subjects received 0.2 mmol/kg of a Gadolinium (Gd) based contrast agent (Magnevist, Berlex), delivered intravenously at a rate of about 5 ml/s into the antecubital vein. Injection of the contrast agent began 1.5 minutes after the start of the scan. The duration of the injection was approximately 4–6 sec. The tissue concentration time curve was calculated using the first 30 images after injection. The average signal of the 40 images prior to injection was used as the baseline signal. The arterial input function was determined in each subject from 4–6 pixels containing a large vessel, typically the middle cerebral artery. The CBF of both gray matter and white matter was determined using the procedure described in our previous publication.
15 White matter CBF does not decline with age, in contrast to gray matter CBF.
16 Cortical CBF was normalized to white matter CBF to offset intraindividual differences in absolute CBF. The left and right insular cortices were outlined in the T1 MRI image corresponding to the first EPI image from the perfusion scan time series (because of its high contrast). Then, the outlined regions of interest were registered to the regional CBF map obtained from our former calculation. The mean regional insular CBF values can be obtained by averaging the pixel values in its corresponding region of interest of the rCBF map. Because autonomic control is lateralized at the level of the insula, left hemisphere dominant asymmetry in the ratio of left:right insular cerebral blood flow (CBF) may define a group at relatively high risk for falls, syncope, or sudden death. Therefore, subjects were classified
a priori into “high” and “low” risk groups on the basis of the ratio of their right:left insular rCBF. The high-risk group included individuals with left dominant (i.e., relatively low right) insular rCBF.
Psychometric Measures
Depressive symptoms were assessed using the 15-item short Geriatric Depression Scale.
17 –
18 Geriatric Depression Scale scores range from 0–15 and higher scores indicate a worse score. A cut-point of 6–7 best discriminates clinically depressed from nondepressed elderly.
19The University of Pennsylvania Smell Identification Test (UPSIT)
20 was used to assess olfaction. The UPSIT is a standardized test of odor identification with good test-retest reliability (r=0.95) and strong correlation with detailed olfactory threshold tests (r=0.80). It contains 40 microencapsulated odors. The subject scratches and sniffs each test strip and chooses among multiple choices for the identity of each odor represented. The prompted design of a multiple-choice format minimizes the likelihood that UPSIT scores will be affected by verbal memory or executive impairments. UPSIT scores ≤ 18 indicate “anosmia” (e.g., severe odor identification deficits); scores of 19–33 (men) and 19–34 (women) indicate “microsmia.” For this study, we assessed left and right nostril performance separately by asking the subject to close the contralateral nostril during UPSIT testing. The ratio of right:left UPSIT performance was then calculated.
Cognitive Measures
General Cognition
The Mini-Mental State Examination (MMSE)
21 is a well-known and widely used test for screening cognitive impairment. Scores range from 0–30, and a score of 28/30 is the median for normal octogenarians of greater than 12 years of education.
21 Scores below 24 reflect cognitive impairment.
Memory Function
The Connecticut Picture Learning Test (COPLT)
22 is a pictorial version of the California Verbal Learning Test. Subjects are asked to recall objects from a set of 16 line drawings of familiar objects (immediate recall), over five consecutive trials (COPLT total, maximum score of 80), after distraction (COPLT short, maximum score of 16) and after a 20 minute timed delay (COPLT long, maximum score of 16). The difference between the short delay trial and the fifth learning trial is COPLT retention (range=0−15).
As in the COPLT, subjects taking the Rey Auditory Verbal Learning Task (RAVLT)
23 are asked to recall words from a 15-word list immediately (immediate recall), over five consecutive trials (RAV total, maximum score of 75), after distraction (RAV short, maximum score of 15), and after a 20-minute timed delay (RAV long, maximum score of 15). The difference between the short delay trial and the fifth learning trial is RAV retention (range=0–15).
Executive Control
An Executive Clock-Drawing Task
24 (CLOX) is a brief executive cognitive function measure based on a clock-drawing task. It is divided into two parts. CLOX1 is an unprompted task that is sensitive to executive control. CLOX2 is a copied version that is less dependent on executive skills. CLOX1 is more “executive” than several other comparable clock-drawing tasks.
25 Each CLOX subtest is scored on a 15-point scale. Lower CLOX scores are impaired. Cut-points of 10/15 (CLOX1) and 12/15 (CLOX2) represent the 5th percentiles for young-adult comparison subjects.
The Executive Interview (EXIT25)
26 provides a standardized clinical executive cognitive function assessment. Items assess verbal fluency, design fluency, frontal release signs, motor/impulse control, imitation behavior, and other clinical signs associated with frontal system dysfunction. EXIT25 scores correlate well with other ECF measures including the Wisconsin Card Sorting Task (WCST) (r=−0.54), Trail Making Test Part B (r=0.64), Lezak’s Tinker Toy test (r=−0.57), and the Test of Sustained Attention (Time, r=0.82; Errors, r=0.83). EXIT25 scores range from 0 to 50. High scores indicate impairment. A score of 10/50 reflects the 5th percentile for young adults. Scores ≥15/50 suggest clinically significant executive cognitive function dysfunction.
Trail Making Test Part B
27 is a test of conceptualization, psychomotor speed, and attention. The subject draws lines to connect consecutively numbered circles on Trails A. Trails B requires the subject to connect consecutively numbered and lettered circles, alternating between the two sequences. The time in seconds to complete each task is recorded.
Visuospatial Function
CLOX2 and Trails A were used as foils to their more executive counterparts, CLOX1 and Trails B. In addition, we used the Weschler Adult Intelligence Scale—Revised (WAIS) Digit Symbol Coding.
28 The Digit Symbol Coding is a test of psychomotor speed and attentional control. The subject is asked to copy, as quickly as possible, nonsense symbols corresponding to specific numbers presented in a key at the top of the page.
Cardiovascular Assessment
Potential subjects with self-reported histories of diabetes, acute myocardial infarction, cerebrovascular accidents, valvular heart disease, or cardiothoracic surgery were excluded. Subjects without self-reported acute myocardial infarction or cerebrovascular accidents were further tested with resting electrocardiography (ECG) and T2 weighted MRI. Five subjects were excluded because of abnormal ECG findings, including silent acute myocardial infarction by ECG, greater than first degree heart block, bundle branch blocks, or atrial fibrillation. Subjects with supraventricular arrhythmias and/or first degree heart block were allowed. Potential subjects with any self-reported history of valvular disease or congestive heart disease were excluded. Echocardiography (2D ECHO) was obtained in a subset of cases with self-reported orthopnea/dyspnea on exertion. No subject had an abnormal left ventricular ejection fraction. At MRI, participants were screened for focal cortical lesions, or lacunar subcortical gray matter lesions. None were excluded on this basis. Subjects with “ischemic” periventricular white matter disease were allowed. Autonomic function in the remaining cases was assessed by ECG and 24 hour Holter monitoring. Mean p -r (PR) intervals and rate corrected Q-T intervals (QTc) were calculated from Holter records. Systolic blood pressures were assessed lying, sitting and standing, by mercury sphygmomanography, using a size appropriate cuff in the right upper extremity. Positional blood pressure changes (ΔSBP) were assessed by the difference between sitting and standing systolic blood pressures.
Statistical Analyses
Cross-group differences were tested by Student’s t test, and Analysis of Variance (ANOVA). Associations between continuous variables were tested by parametric correlations. Significant univariate correlations were adjusted in multiple regression models. Because of the small sample size, regression models were limited to one covariate.
RESULTS
Left and right insular rCBF were highly intercorrelated (r=0.97, p<0.001). Each was also strongly correlated with both total white matter and frontal cortical rCBF (r=0.80–0.83, p<0.001 in all cases). Mean resting insular rCBF was significantly higher on the right (t test for dependent samples: t=2.5, p=0.02) and the ratio of right:left insular rCBF was skewed in favor of right insular dominance. On the other hand, 10/29 participants (34.5%) had left dominant rCBF and were assigned to the theoretically “high” risk group, defined by relatively low right insular rCBF.
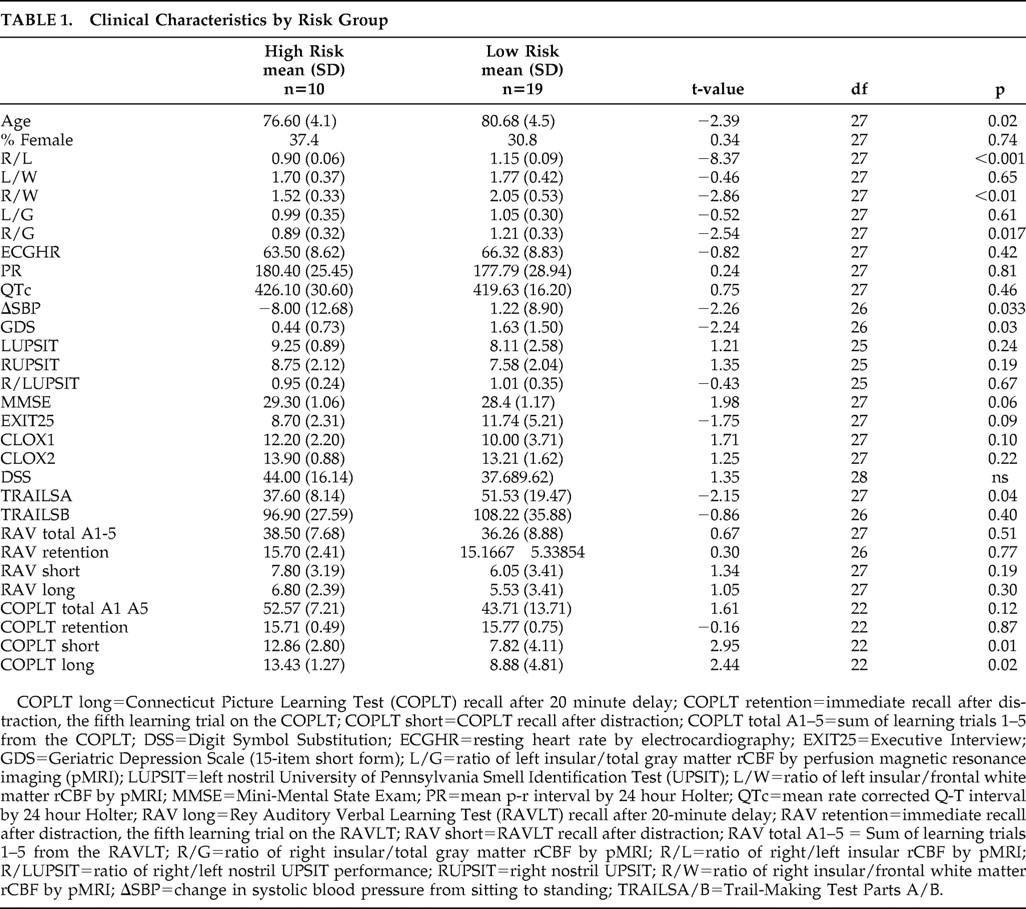
Clinical data, stratified by “risk” group, are presented in
Table 1 . There were no differences in left insular, frontal or white matter rCBF across high/low risk groups. In contrast, right insular rCBF was significantly lower in high-risk group (F=6.5, df=1, 28, p=0.01). The ratio of right insular to frontal rCBF was significantly lower in the high-risk group (t=−2.54, df=27, p=0.017). There was no difference in the ratio of left insular to frontal rCBF. Similarly, the ratio of right insular to total white matter rCBF was significantly lower in the high-risk group (t=−2.86, df=27, p<0.01). There was no difference in the ratio of left insular to total white matter rCBF. These findings suggest an absolute reduction in right insular rCBF in high-risk cases.
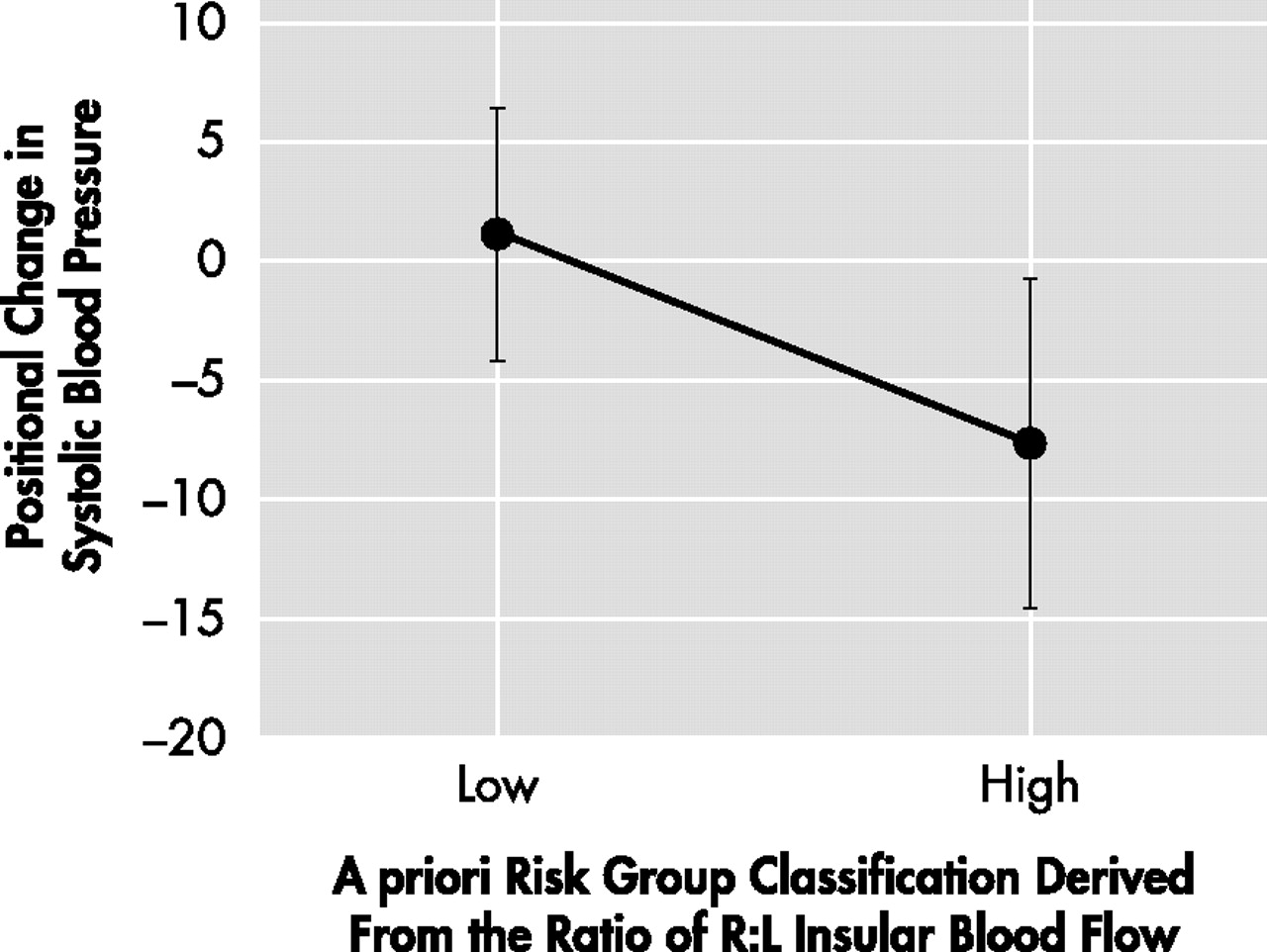
The high-risk group also had a greater positional drop in blood pressure (F=4.9, df=1, 27, p=0.04) (
Figure 1 ). There were no cross-group differences with regard to resting heart rate, mean 24 hour P-R interval, or mean 24 hour rate corrected Q-T interval. The high-risk group was significantly younger (t=−2.39, df=27, p=0.02), and had fewer depressive symptoms on the Geriatric Depression Scale (t=−2.24, df=26, p=0.03). Neither group approached the threshold for clinically significant Geriatric Depression Scale scores. High-risk subjects performed significantly better on the COPLT (short: t=2.95, df=22, p=0.01; long: t=2.44, df=22, p=0.02), but not the RAVLT. The high-risk group also performed less well on Trails A (t=−2.15, df=27, p=0.04). There were no cross-group differences on measures of general cognition (MMSE) or executive function (CLOX1, EXIT25, Trails B) and the mean scores were in the normal range on all of these measures. Positional blood pressure change was significantly associated with memory, as measured by the COPLT (total: r=−0.44, p<0.05; short: r=−0.49, p<0.05; long: r=−0.44, p<0.05). However, these associations were all inverse, suggesting that positional drops in blood pressure were associated with better memory performance. These associations resisted adjustment for age. However, no memory measure predicted positional blood pressure change after adjusting for right:left insular rCBF (nonsignificant), suggesting that asymmetric insular function mediates their association. Positional blood pressure change was not significantly correlated with RAVLT scores, CLOX1, CLOX2, DSS, EXIT25, MMSE, Trails A/B, or UPSIT scores. The ratio of right:left insular rCBF was also significantly associated with COPLT and not RAVLT performance, (COPLT total: r= −0.46, p<0.01; COPLT short: r= −0.68, p<0.01; COPLT long: r= −0.70, p=0.005). These associations were again inverse, suggesting that asymmetric reductions in right insular rCBF predicted better memory performance. Conversely, asymmetric reductions in left insular rCBF would be associated with worsened memory performance. The associations between right:left insular rCBF, COPLT short, and COPLT long resisted adjustment for age.
DISCUSSION
We have demonstrated a significant association between the ratio of right:left insular rCBF and (1) positional blood pressure changes, and (2) cognitive test performance, especially on memory measures, in a small sample of elderly persons, free of comorbid diabetes mellitus or cardiovascular disease. Moreover, these associations can be demonstrated in the absence of clinically significant cognitive impairment. These data support our hypothesis that a subset of nondemented elderly persons (35% of this sample) exhibit relatively reduced right insular rCBF. This asymmetry appears to be related to absolute reductions in right insular rCBF rather than relative increases in left insular rCBF. Since autonomic control is lateralized in humans at the level of the insula this may have implications for autonomic related morbidity and mortality in the elderly. Hilz et al.
6 have demonstrated positional drops in systolic blood pressure and augmentation of “high frequency” spectral power density in interbeat
R -R heart rate variability among epileptic subjects undergoing right hemisphere cortical functional inactivation as part of their perioperative assessments. As in this study, asymmetric right hemisphere dysfunction was associated with a drop in positional blood pressure, but not resting heart rate, and the absolute value of the positional changes was somewhat less than that which is felt to be clinically “meaningful.” Nonetheless, high frequency spectral power has been previously associated with parasympathetic modulation
29 and is a significant predictor of mortality.
30The cause(s) for an absolute reduction in right insular rCBF among the high-risk group cannot be determined from these data. However, it is not likely to be due to ischemic lesions, as these participants were carefully screened for ischemic cardiovascular disease, and did not exhibit gross ischemic cerebrovascular disease in their T2 weighted MRI scans. On the other hand both Alzheimer’s disease and Lewy Body Disease are associated with insular lesions
31 and Lewy Body Disease is associated with greater visuospatial cognitive impairment,
32 accelerated mortality,
33 and greater fall risk
34 than Alzheimer’s disease.
We are currently testing the hypothesis that insular neurodegenerative changes are associated with autonomic dysfunction among decedents from the Honolulu-Asia Aging Study. Our preliminary data suggests that the associations between age, QTc intervals and survival are moderated by insular neurofibrillary tangles counts.
12 Insular involvement by the Alzheimer’s disease process would require a Braak stage of at least II-III.
13 These Braak stages are consistent with normal cognition/mild cognitive impairment
35 and thus with the range of test scores we observed in this sample.
However, we have unexpectedly found the high-risk group to be younger and generally less memory impaired that those in the low risk group. Similarly, memory impairment, even on the COPLT, was inversely related to the observed asymmetric reduction in right insular rCBF and positional changes in blood pressure. There might be several reasons for this. First, this finding may be spurious. Our sample size is small and multiple comparisons favor spurious associations. On the other hand, we previously observed a specific association between Trails A and survival.
2 This association did not extend to Trails B or comparable executive measures (e.g., CLOX1 or the EXIT25). Similarly, the high-risk group in this study performed significantly worse on Trails A, but neither Trials B, CLOX1, or the EXIT25, consistent with our previous results.
Second, the insular mediated positional changes we observed might be due to medication effects not considered here. However, this does not seem likely. First, medications would be expected to affect the insula bilaterally, as has been shown for antidepressants
36 and antipsychotics.
37 Second, certain medications that might be expected to affect both memory and autonomic control (e.g., anticholinergics and antidepressants), would be expected to favor a positive association between memory impairment and positional changes in systolic blood pressure. The use of acetylcholinesterase inhibitors among memory impaired subjects might explain an inverse association between the severity of memory impairments and orthostasis, as the latter is a known side effect of this class.
Finally, it may be that memory tasks, even visual memory tasks such as the COPLT, depend on left mesiotemporal regions of interest. In fact, surprisingly little is known about the CNS correlates of many cognitive measures, and even clock-drawing tasks can be associated with left hemisphere regions of interest.
38 –
39 Lye et al.
40 report significant associations between left, not right, hippocampal atrophy and a visuospatial memory task in a large nondemented elderly sample of similar age and MMSE performance to our own. Similarly, Stern et al.
41 report left anterior insular activation during a verbal memory task. Thus, poor memory performance in our subjects may reflect asymmetric Alzheimer’s disease pathology involving the left hemisphere, favoring a low risk presentation. This would explain our failure to associate memory measures with mortality in elderly retirees.
2In summary, a substantial fraction of well elderly persons free of clinically significant cardiovascular disease can demonstrate asymmetric resting insular rCBF by pMRI. This has implications for autonomic related morbidity and mortality in the elderly. A greater positional drop in blood pressure can be demonstrated in association with left>right insular rCBF, and absolute reductions in right insular rCBF. These changes can be demonstrated in the absence of dementia. Although these data cannot address the cause of these asymmetries in insular rCBF, preclinical Alzheimer’s disease pathology is endemic in the nondemented elderly, involves the insula, and is likely to be unilateral in its earlier stages.