A pathy is one of the most frequent behavioral abnormalities in patients with Alzheimer’s disease, and is significantly associated with more severe impairments in activities of daily living and cognitive deficits,
1 poor awareness of behavioral and cognitive changes, and increased stress for families.
2 We reported a significant association between more severe apathy and larger white matter hyperintensities in a study that used MRI and a semiquantified technique to measure white matter hyperintensities.
3 Studies using single photon emission computed tomography (SPECT) reported a significant association between apathy and low perfusion in prefrontal, anterior temporal, temporoparietal, and cingulate regions.
4 –
7 This variability may be related to the different methods used to diagnose apathy, which primarily consisted of arbitrary cutoff scores on ad hoc apathy rating scales. Another confounding factor is that frequent comorbid conditions of apathy in Alzheimer’s disease, such as depression, were not considered for analysis.
There are few neuroimaging or neurophysiological studies of depression in Alzheimer’s disease. Using SPECT we found that Alzheimer’s disease patients with major depression had a significantly lower blood perfusion in left temporal and parietal regions than nondepressed Alzheimer’s disease patients.
8 Using quantified electroencephalography (qEEG) we found that depressed Alzheimer’s disease patients had a significantly higher percentage of theta activity in posterior brain areas than nondepressed patients with Alzheimer’s disease.
9 We also reported a significant association between depression (with or without Alzheimer’s disease) and a significantly lower alpha relative power in the right posterior brain region as compared to nondepressed individuals with or without Alzheimer’s disease.
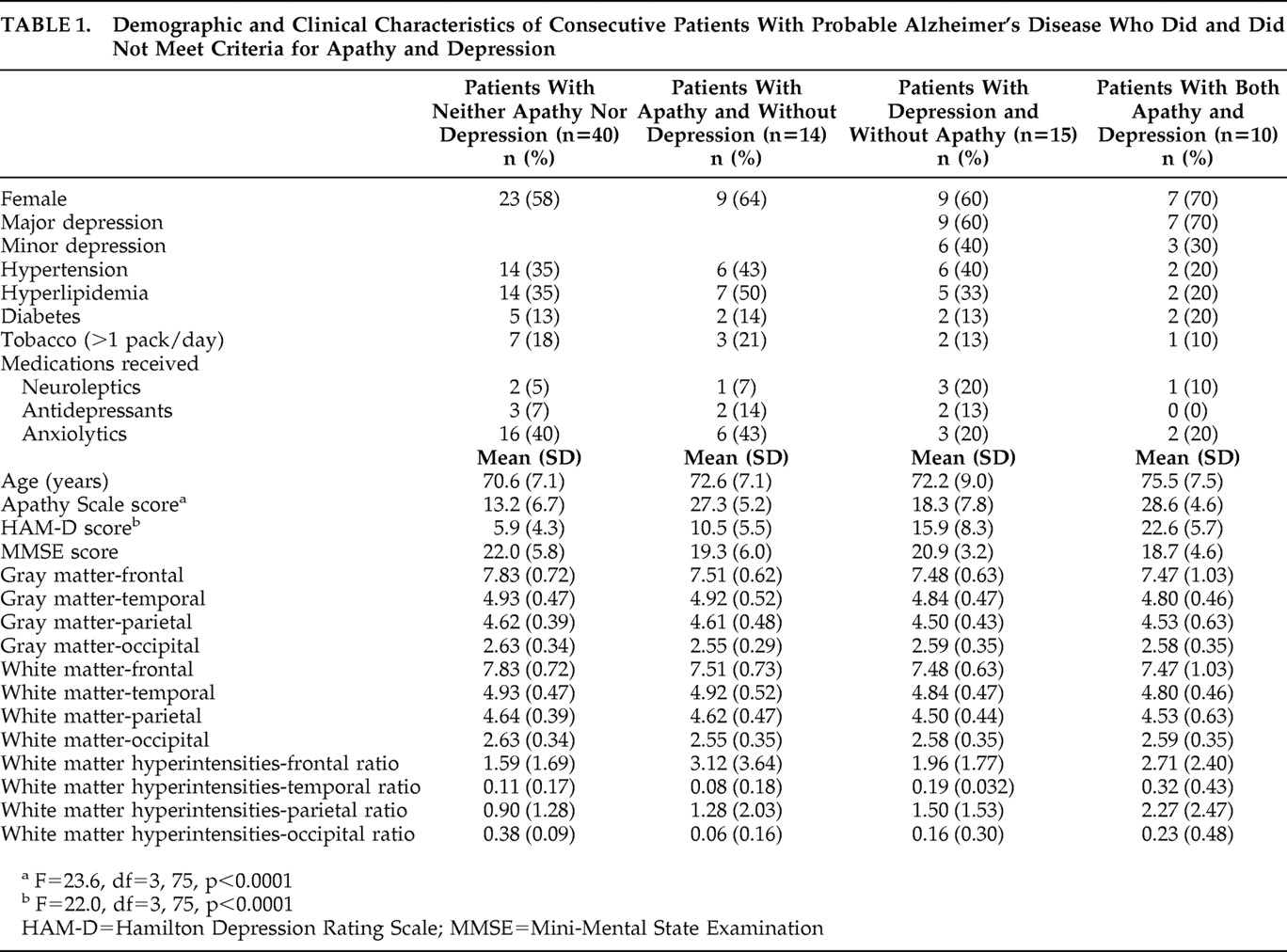
10For the present study we examined the association between apathy, depression, and structural brain changes in a consecutive series of 79 patients with mild, mild, or moderate Alzheimer’s disease. All patients were assessed for both apathy and depression using a structured psychiatric interview and standardized diagnostic criteria. Gray matter, white matter, and white matter hyperintensity-lobar volumes were measured using a semiautomated volumetric and fully quantified technique. Based on our previous findings, we hypothesized that Alzheimer’s disease patients with apathy would show a relatively larger volume of frontal white matter hyperintensities and frontal cortical atrophy than Alzheimer’s disease patients without apathy and that Alzheimer’s disease patients with depression would show similar abnormalities in right posterior brain regions.
DISCUSSION
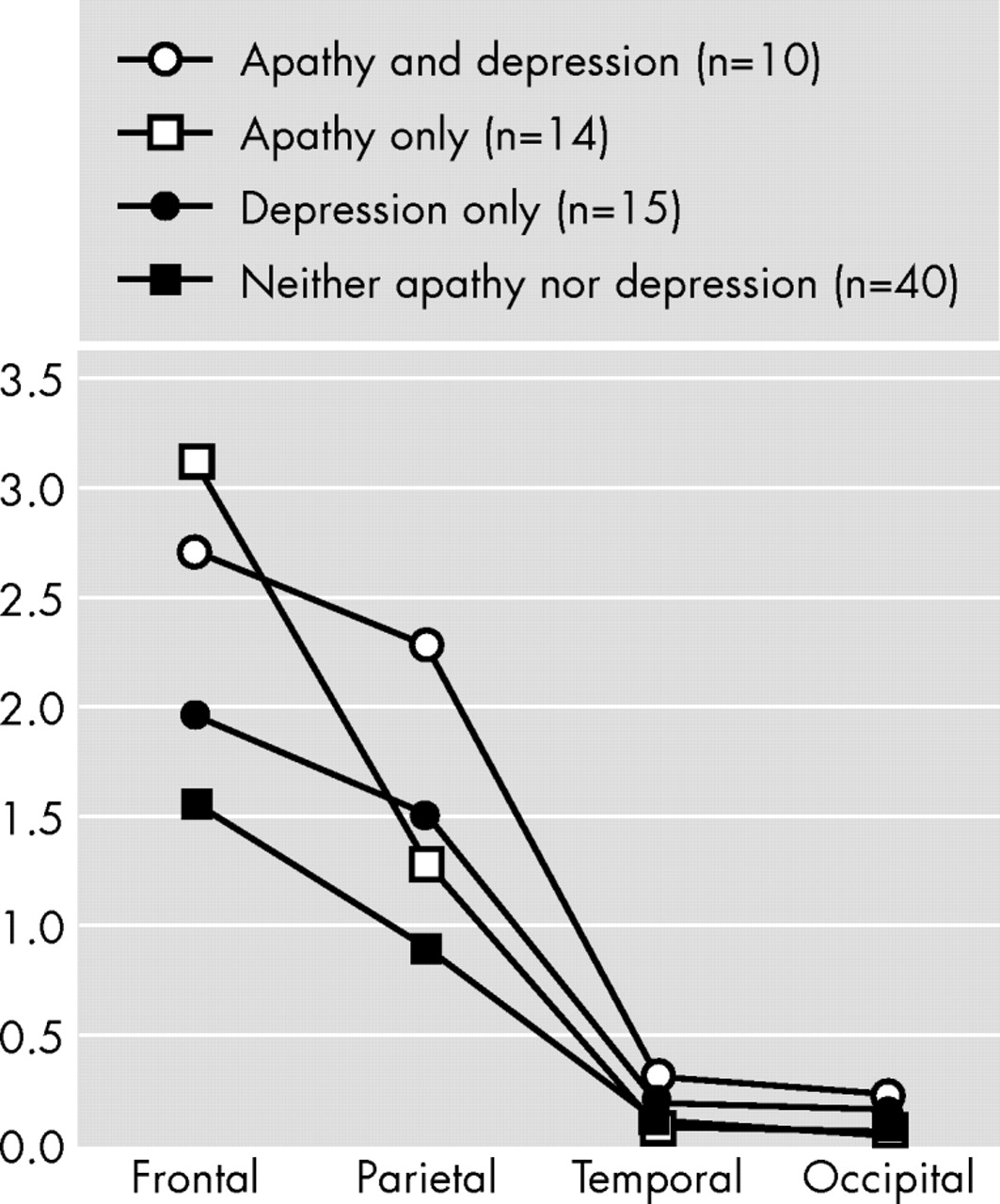
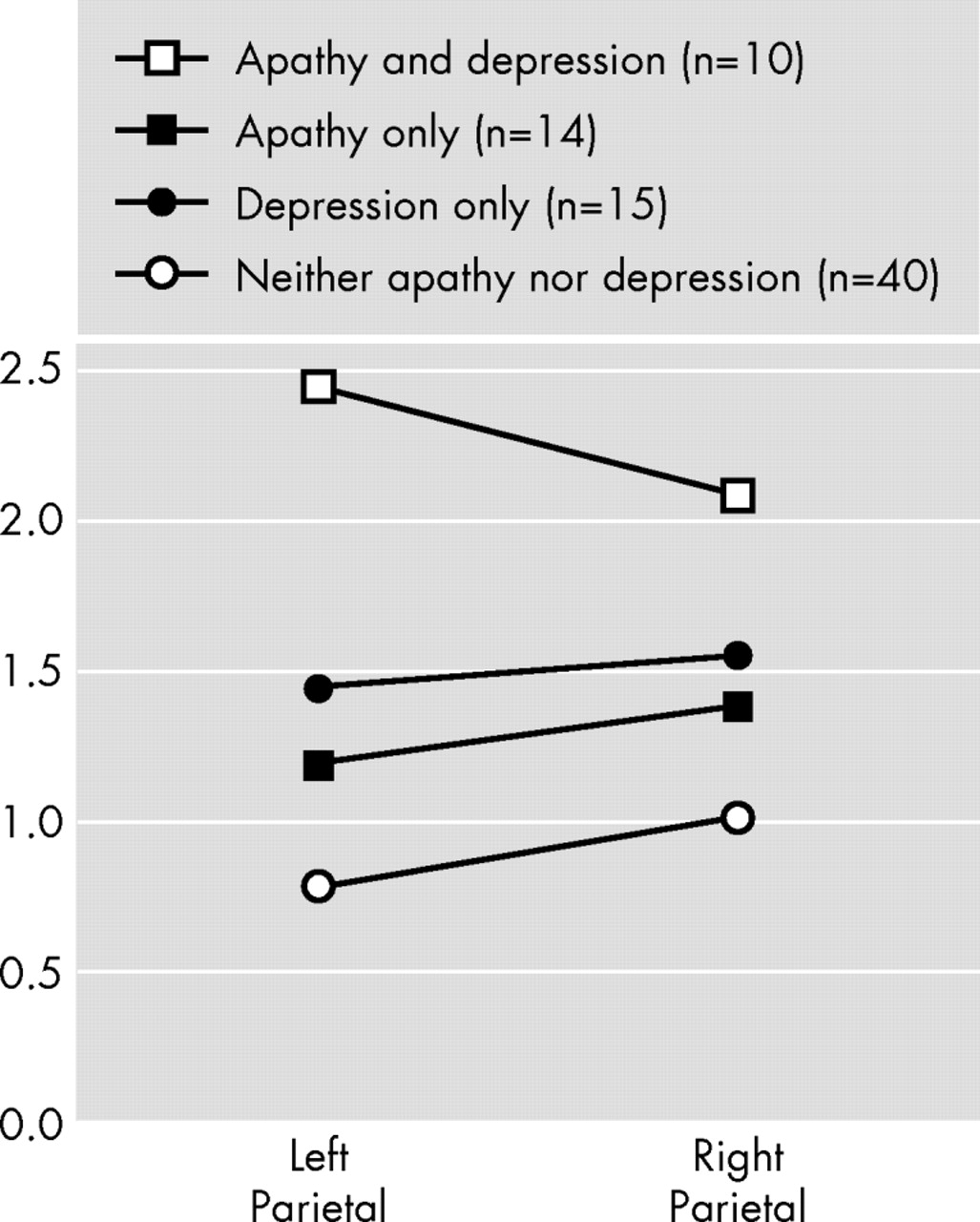
We examined the association between apathy, depression, and brain structural findings in a consecutive series of patients with Alzheimer’s disease and no focal brain lesions, and there were several important findings. First, apathy was significantly associated with a larger white matter hyperintensity volume in the frontal lobes. Second, depression was significantly associated with a larger white matter hyperintensity volume in the right parietal lobe. Finally, there were no significant associations between apathy or depression and gray matter or white matter lobar volumes.
Before further comments, several limitations of our study should be pointed out. First, temporal and occipital white matter hyperintensity volumes were small, and our study may be underpowered to detect significant between-group differences on these structures. Second, while the difference was not statistically significant, nondepressed patients had a higher intake of anxiolytics than depressed patients. Depression was clearly undertreated in our patients, which is congruent with findings from other groups.
19 Third, our brain segmentation method was based on Talairach’s voxels and was restricted to gray matter, white matter, and white matter hyperintensities volumes in brain lobes. A study by Apostolova et al.
20 demonstrated significant correlations between increased apathy scores and decreased gray matter volume in the bilateral anterior cingulate and left mesial frontal cortex. Future studies may use lobar segmentation and recently developed techniques (e.g., diffusion tensor imaging) to examine associations between behavioral disorders and hyperintensities in specific white matter tracts.
21,
22 However, to our knowledge this is the first study to examine associations between specific brain structural changes and both apathy and depression in dementia using a semiautomatic and fully quantified method, and to use standardized clinical diagnoses for both apathy and depression. In a recent study, Staekenborg et al.
23 found no association between white matter hyperintensities and behavioral and psychological symptoms in a series of 111 patients with Alzheimer’s disease. The degree of white matter hyperintensity severity was rated visually, and most patients had no or mild psychiatric symptoms. The authors suggested using quantitative techniques to measure white matter hyperintensities and to examine the association between regional white matter hyperintensities and specific psychiatric disorders.
Whereas apathy is one of the most frequent and severe behavioral changes in Alzheimer’s disease, the concept of apathy (i.e., whether apathy is a symptom or a syndrome) is still not clear, and this condition has been diagnosed using different strategies. We recently validated Marin’s
24 conceptual construct of apathy into standardized criteria.
16 We used this diagnostic scheme in a study that included 319 patients with Alzheimer’s disease and found apathy to be significantly associated with relatively more severe impairments in activities of daily living and cognitive functions, older age, and poor awareness of behavioral and cognitive changes relative to patients without apathy. The present finding of a significant association between apathy and a larger frontal white matter hyperintensities volume provides further validation to our diagnostic criteria.
We now question the putative role of frontal white matter hyperintensities in the mechanism of apathy. Pathological studies demonstrated that white matter hyperintensities are related to different severity of myelin degradation, loss of axons and oligodendroglia, gliosis, disruption of the ependymal lining, and/or infarction.
25 –
27 Recent studies suggest that white matter hyperintensities are associated with reduced blood perfusion in brain areas without white matter hyperintensities such as the basal ganglia and thalamus.
28 –
31 Cummings
32 suggested that apathy in dementia results from damage to a frontal-subcortical circuit that normally mediates complex cognitive functions and motivation. Our findings provide support to Cummings’ hypothesis and suggest that frontal white matter hyperintensities could produce apathy by disrupting deep white matter afferents and efferents to the basal ganglia and/or by decreasing metabolic activity in frontal subcortical regions.
Among nondemented individuals, increased white matter hyperintensity severity was associated with new onset and a more chronic course of depression and poor response to antidepressant medication.
33,
34 Several studies reported a significant association between greater white matter hyperintensities in frontal brain regions and depression.
34 In this study we found that patients with depression had a significantly larger white matter hyperintensity volume in the right parietal lobe than patients without depression, and no significant association was found between depression and frontal white matter hyperintensities. In a study that examined quantified electroencephalographic (qEEG) correlates of depression in Alzheimer’s disease, we found a significant association between depression and increased theta power in posterior brain areas.
9 In a subsequent study we compared 17 Alzheimer’s disease patients with comorbid depression with 13 age-matched individuals with depression, but no dementia and 10 age-matched healthy comparison subjects.
10 While depression (with or without Alzheimer’s disease) was significantly associated with lower alpha relative power, among Alzheimer’s disease patients this change was restricted to posterior brain areas. Furthermore, Alzheimer’s disease patients with depression also showed significant increases in delta power in posterior brain areas as compared to depressed individuals without dementia. Using 99mTcHMPAO SPECT, we compared 10 Alzheimer’s disease patients with major depression with 21 Alzheimer’s disease nondepressed patients. The main finding was that patients with major depression had significant hypoperfusion in temporoparietal regions as compared to the nondepressed group.
8 Among 93 patients with a right hemisphere stroke, we found a significant association between depression and right parietal damage.
35 Future studies should compare the severity and location of white matter hyperintensities in comparable samples of demented and nondemented individuals with depression.
In conclusion, we found a significant association between white matter hyperintensities and behavioral disorders in patients with Alzheimer’s disease. Whereas apathy was significantly associated with larger white matter hyperintensities in the frontal lobes, depression was associated with larger right parietal white matter hyperintensities. On the other hand, neither gray nor white matter atrophy was associated with apathy or depression. Future studies may use more sophisticated neuroimaging techniques to further examine the association between pathological white matter changes and the behavioral disorders of dementia.