I nvoluntary emotional expression disorder (IEED) is characterized by sudden episodes of laughing or crying that either occur spontaneously or are out of proportion to the stimuli that provoke them. A variety of terms have been used to refer to this disorder, including pseudobulbar affect, pathological crying and laughing, emotional incontinence or lability, and pathologic affect.
1,
2 Recently, Cummings et al.
3 proposed the term involuntary emotional expression disorder (IEED) as an inclusive nosological concept to characterize patients with neurological disease or injury who experience episodic and involuntary bouts of uncontrollable emotional expression.
IEED has been observed in patients with stroke (10–20%),
4 –
9 traumatic brain injury (5–11%),
4,
5 Alzheimer′s disease (39%),
10 multiple sclerosis (10%),
11 amyotrophic lateral sclerosis (19–49%),
12,
13 seizure disorders,
14 multiple system atrophy-cerebellar type,
15 and corticobasal degeneration.
16 However, to our knowledge there are no previous reports on the frequency and clinical correlates of IEED in Parkinson’s disease.
Classic pathophysiological theories of IEED are based on the assumptions of serial processing and hierarchical control. According to these assumptions, IEED results from the release of cortical inhibition of brainstem centers that integrate the motor activation patterns involved in laughing and crying. Thus, IEED is an essential part of the pseudobulbar palsy syndrome associated with bilateral lesions in corticobulbar pathways. However, IEED may also be seen in patients with unilateral lesions that do not involve motor or premotor areas. For instance, Ross and Rush
17 reported that IEED may result from lesions of the right inferior frontal lobe in association with a major depressive disorder. More recently, Parvizi et al.
15 suggested that the critical lesion or dysfunction eliciting IEED is located along fronto-ponto-cerebellar pathways.
18 IEED can be seen in neurological disorders without any demonstrable lesions but with disruptions in fronto-subcortical circuits. For instance, McCullagh et al.
13 studied amyotrophic lateral sclerosis patients and implicated the prefrontal cortex in the pathophysiology of IEED.
There is also evidence that IEED is related to disruption of monoaminergic modulation of both limbic structures and bulbar motor networks.
19 If this hypothesis is correct, IEED should be a relatively frequent finding among patients with Parkinson’s disease, who undergo a selective degeneration of aminergic pathways and brainstem nuclei.
6,
7,
18,
20In spite of Parkinson’s disease being the second most common neurodegenerative disease, little is known about IEED in Parkinson’s disease. Thus, the aim of the present study was to examine the frequency and clinical correlates of IEED in Parkinson’s disease. We hypothesized that IEED would be relatively common among Parkinson’s disease patients, IEED would occur without comorbid depression, and IEED would be associated with excess disability.
METHODS
Patients
We assessed a series of 131 patients meeting the United Kingdom Parkinson’s Disease Society Brain Bank
21 clinical criteria for idiopathic Parkinson’s disease who were followed at a general neurology clinic of a tertiary care hospital in Buenos Aires, Argentina. After the methodology of the study was fully explained, an informed written consent was obtained from all the participants. Patients were assessed by a neurologist who was blind to the psychiatric data and a psychiatrist blind to neurological findings.
In order to examine as homogeneous a group of Parkinson’s disease patients as possible, patients with radiological evidence of cerebrovascular lesions (i.e., MRI scans revealing focal lesions that were hypointense in T1 and hyperintense in FLAIR sequences and were greater than 3 mm along their major axis in transversal views) were excluded from the study. Other exclusion criteria were a history of cognitive decline starting 1 year or less from the onset of parkinsonian signs, a history of neuroleptic medication exposure, and lack of therapeutic response to antiparkinsonian drugs.
Neurological Examination
The neurological examination was conducted by a neurologist blinded to the neuropsychiatric results. Parkinson’s disease symptoms were rated with parts I-IV of the Unified Parkinson’s Disease Rating Scale (UPDRS),
22 and overall severity of Parkinson’s disease was rated with the Hoehn and Yahr Staging Scale.
23 Higher scores indicate greater severity of impairment on both instruments.
Psychiatric Examination
The Pathological Laughing and Crying Scale
19 is a reliable and valid interviewer-rated scale that rates severity of IEED symptoms (higher scores indicating greater severity), including the frequency of crying or laughing episodes, duration and degree of voluntary control of episodes, their relationship to triggering events, inappropriateness in relation to prevailing mood, and degree of resultant distress. The scale is administered to the patient with at least one first-degree relative or caregiver serving as an informant. The time frame for including symptoms was established
a priori as the 4-week period preceding the assessment.
Diagnosis of Involuntary Emotional Expression Disorder
Patients were considered to have IEED if they met all of the three following criteria: they scored 2 or 3 (i.e., more impaired) on item 2 of the Pathological Laughing and Crying Scale assessing the frequency of crying episodes; they scored 2 or 3 on item 13 of the Pathological Laughing and Crying Scale assessing loss of voluntary control of emotions during episodes; and they scored 2 or 3 on item 18 of the Pathological Laughing and Crying Scale assessing distress and embarrassment associated with the episodes. In addition, a total score ≥10 was required for a patient to be diagnosed as having IEED.
The Structured Clinical Interview for DSM-IV (SCID),
8 a semistructured interview, was used to assess the presence of axis I DSM-IV disorders. The SCID was conducted with the patient and an informed other in frequent contact with the patient (i.e., at least 3 times per week during the past 6 months). The Hamilton Depression and Anxiety Rating Scales (HAM-D, HAM-A)
8,
9,
24 were used to assess the severity of depression and anxiety symptoms, respectively. Higher scores on both scales indicate greater severity of symptoms.
The Apathy and Irritability Scales
25,
26 are interviewer-rated scales that assess severity of apathy and anxiety, respectively. Scores were based on information obtained from the patient and a relative or caregiver. For both instruments, higher scores indicate greater severity of symptoms. The Overt Aggression Scale
27 measures the severity of four specific aspects of aggressive behavior (i.e., verbal aggression, and physical aggression against self, objects and other people). It is an interviewer-rated scale based on observable criteria and information obtained from the patient and an informed other. The Hachinski Ischemic Scale
28 assesses the contribution of cerebrovascular disease to the etiology of dementia. Higher scores indicate increased severity of cardiovascular risk factors.
Mini-Mental State Examination (MMSE)
29 was used as a measure of global cognitive abilities. The Functional Independence Measure
30 assesses patients’ level of functioning in basic and instrumental activities of daily living (e.g., self-care, sphincter control, mobility, locomotion, communication, and social cognition). Higher scores indicate greater independence in performing activities of daily livings.
Statistical Analysis
The Wilcoxon-Mann-Whitney U test was use to compare group means, as much of the data did not meet the homogeneity of variance and normality assumption required by the standard t test. Frequencies between groups were compared using Fisher’s exact test. We used simple linear regression to control for covariates. Since the residual analysis in each case was satisfactory, no further transformations of the data were necessary. All p values reported are two-tailed, and significance level was set at 0.05.
RESULTS
Frequency of IEED and Demographic Variables
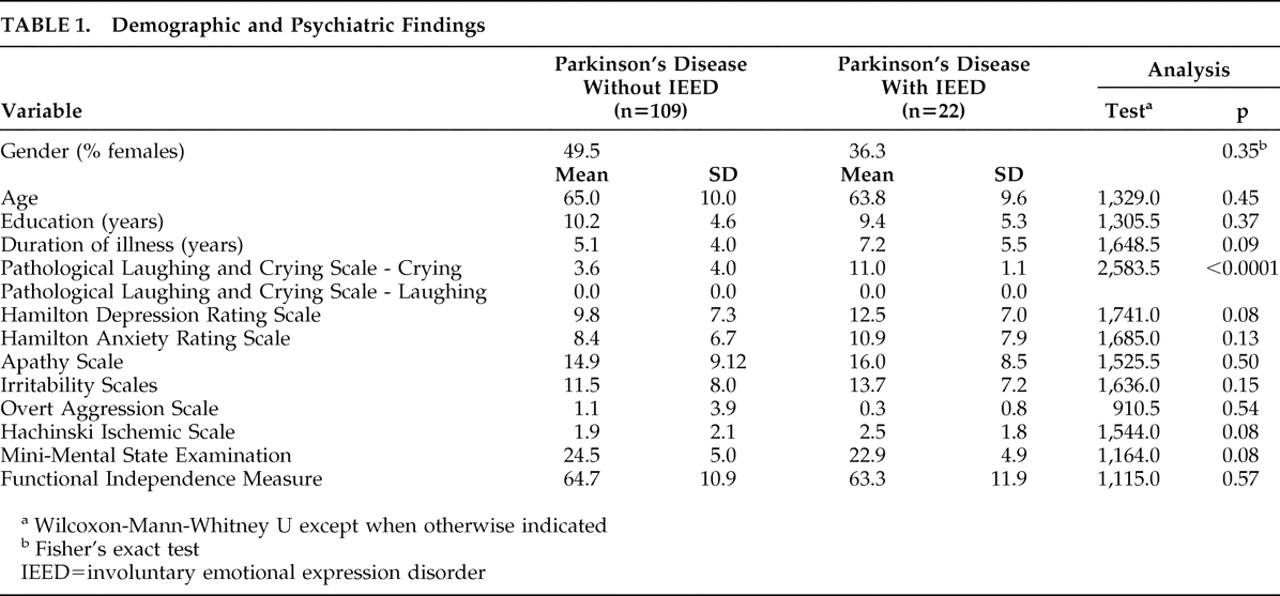
Twenty-two (16.8%) patients had active IEED, all of them with crying episodes only (we did not observe cases of pathological laughter). These 22 patients constituted the IEED group, whereas the rest (n=109) of the Parkinson’s disease patients were categorized as the non-IEED group. There were no between-group differences in demographic variables (
Table 1 ).
Psychiatric Variables
Eleven (50.0%) of the IEED patients met DSM-IV criteria for a depressive disorder (major depression [n=7] or dysthymia [n=4]). In the non-IEED group, 48 (44.0%) met DSM-IV criteria for depression (major depression [n=25] or dysthymia [n=23]). There were no differences between the IEED and the non-IEED groups in the frequency of major depression or dysthymia. In addition, there were no between-group differences in the severity of anxiety symptoms, apathy, irritability, or aggressiveness (
Table 1 ).
Patients received antidepressants to reduce depressive symptoms. Benzodiazepines and/or atypical neuroleptics were indicated to treat anxiety, agitation, or psychotic symptoms. However, there were no significant differences between the IEED and the non-IEED groups in the percentage of patients taking this type of medications (
Table 2 ).
Parkinson’s Disease-Related Variables
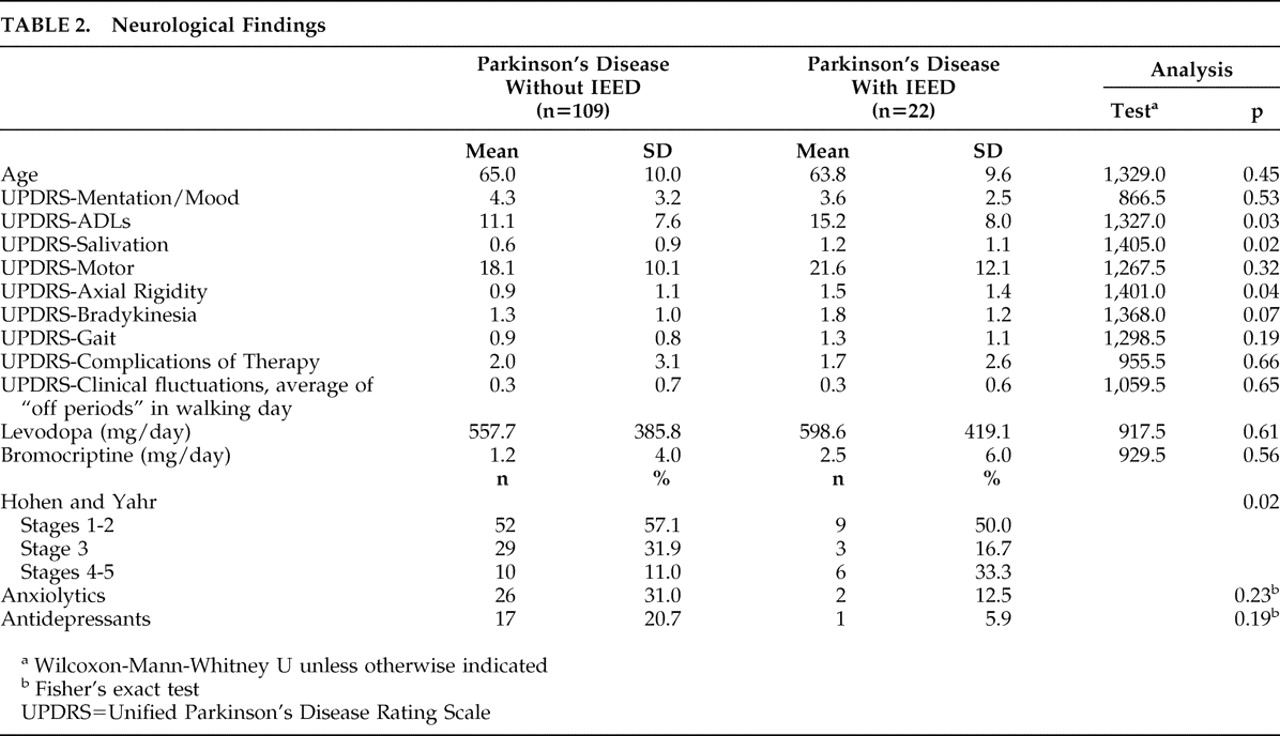
Of the 22 patients with IEED, seven patients (33.3%) were classified as having severe Parkinson’s disease (Hoehn and Yahr stages 4 or 5), compared with 12 of 109 patients (11.0%) without IEED (p=0.02).
Patients with IEED had greater UPDRS salivation (p=0.02), axial rigidity (p=0.04), bradykinesia (p=0.07), and gait disturbance (p=0.19) scores than patients without IEED (
Table 2 ). In addition, the IEED group showed significant functional impairment in the UPDRS activities of daily living section (
Table 2 ). In a linear regression model that included IEED status, duration of illness, and severity of depression, IEED status no longer predicted UPDRS-activities of daily living score. Moreover, there were no significant differences between the IEED and the non-IEED groups in any other clinical variable (Tables
1 and
2 ).
IEED and Comorbid Depression
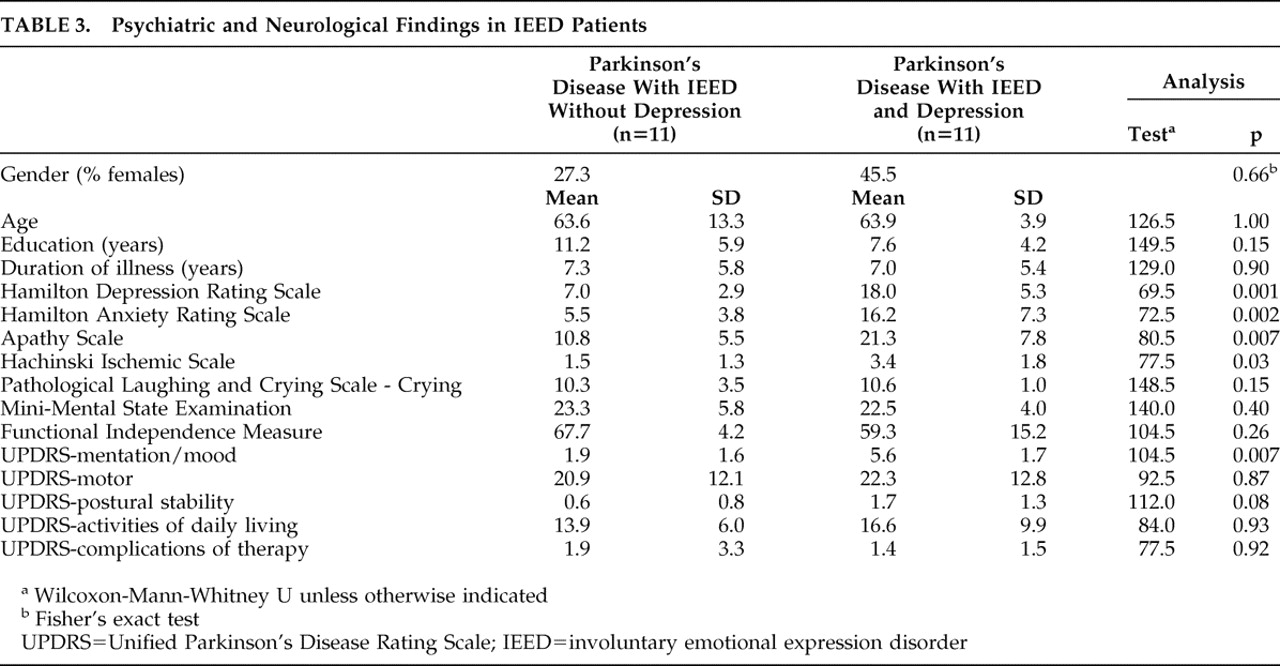
When comparing IEED patients with (n=11) and without depression (n=11), depressed IEED patients had significantly higher HAM-D, HAM-A, and Apathy Scale scores (
Table 3 ). There were no significant between-group differences in Overt Aggression Scale or Irritability Scale scores.
Patients with IEED and depression had significantly higher Hachinski Ischemic Scale scores (
Table 3 ) than nondepressed IEED patients. There were no significant differences between the two groups in any non-mood UPDRS item, Functional Independence Measure, or MMSE scores (
Table 3 ).
DISCUSSION
Involuntary emotional expression disorder (IEED) was present in 16.8% of Parkinson’s disease patients followed in a general neurology clinic at a tertiary metropolitan hospital. Excluding Parkinson’s disease patients with a comorbid depressive disorder, the frequency of IEED was 15.3%. The proportion of patients in advanced stages of the disease was significantly greater among patients with IEED. A greater impairment in activities of daily living was found in the IEED group measured by the UPDRS activities of daily living. However this was not detected by the Functional Independence Measure. The UPDRS activities of daily living is a more sensible and specific measure of activities of daily living impairment than the Functional Independence Measure among patients with Parkinson’s disease. In addition, its high longitudinal reproducibility values make the former a precise measure of change. A 2-point increase in this scale is considered clinically meaningful. Thus, the 4-point difference between the means of the IEED and the non-IEED groups is suggestive of greater disability among patients with IEED.
31 However, there were no other between-group differences in demographic or clinical variables.
Before further discussion of these findings, we need to point out the limitations of our study. Patients included in this study were enrolled in a tertiary care center and might not be representative of other Parkinson’s disease groups. However, all our patients met stringent clinical criteria for Parkinson’s disease, including a positive response to
L -dopa therapy. The neuropsychological evaluation of these patients was limited to a single global cognitive measure (i.e., the MMSE). A more extensive neuropsychological battery would be necessary to examine more subtle cognitive correlates of IEED. Although the difference is not statistically significant, the proportion of patients taking antidepressants was higher among patients without IEED. As antidepressants have been used to treat emotional dysregulation (usually in a lower dose than that used to treat clinical depression), our results might be biased, leading to an underestimation of the frequency of IEED. Another limitation is the exploratory nature of this study and the consequent lack of power to detect significant differences. Finally, the recent definition and diagnosis criteria for IEED were not available at the time of data collection.
3Given these limitations, IEED appears to be relatively common in Parkinson’s disease, particularly in latter stages of the illness. Although IEED can co-occur with depression, it occurred just as frequently in Parkinson’s disease patients without depression, suggesting that this form of emotional dysregulation is distinct from depression and should be included in the differential diagnosis of emotional disturbances associated with Parkinson’s disease.
6Patients with Parkinson’s disease might have coexistent ischemic lesions in the deep hemispheric white matter that might contribute to a rapidly progressive course and cognitive decline.
32 –
34 It is plausible that these ischemic lesions could also contribute to the onset of IEED. However, we took special care to exclude Parkinson’s disease patients with MRI evidence of focal ischemic lesions and/or extensive white matter disease, suggesting that IEED may occur in the absence of coexistent ischemic lesions.
IEED may also occur associated with impairment in the functional integrity of the prefrontal cortex among patients with amyotrophic lateral sclerosis
13 and multiple sclerosis.
11 Recently, Woolley et al.
35 reported the case of a patient with gradually progressive frontal atrophy who was selectively impaired in emotional expression and autonomic reactivity. The left inferior frontal gyrus was the area that showed the most important atrophic changes. Interestingly, this prefrontal area has been shown to have reduced glucose metabolic rates among patients with depression and Parkinson’s disease.
36 Further studies will need to examine the role of neural circuits involving the ventral aspects of the prefrontal cortex in emotional expression of Parkinson’s disease patients, including whether or not the left prefrontal cortex has an inhibitory effect.
Acknowledgments
We thank Drs. Marcelo Merello, Eran Chemerinski, and Janus Kremer for data collection. We especially thank Dr. Sergio E. Starkstein for his valuable advice and comments on the manuscript.