W ith drug therapy innovations many individuals are now living and coping with HIV as a chronic illness. Frequent complaints of persistent fatigue (20%–40%); cognitive impairments (40%–50%); and psychiatric illness, especially depression and anxiety (5%–35%), impose functional limitations in everyday activities and adversely impact quality of life.
1 –
3 Management of these clinical symptoms remains challenging given the complexity of potential factors, both biological and psychosocial, that may underlie the differential expression across individuals. While past research in HIV/AIDS has been invaluable at identifying the contribution of cytokine and endocrine mediators at a cellular mechanistic level in the pathogenesis of HIV/AIDS, and looking at global indicators of disease progression,
4 –
7 better understanding of the variability in symptom manifestation likely requires interdisciplinary research investigating how alterations in biological processes may affect the development and treatment of specific clinical symptoms.
Advancements in psychoneuroimmunology have led to renewed interest in roles that cytokines have in modulating neurotransmitter systems within the brain and thus influencing emotions and cognition. Complaints of fatigue associated with abnormal sleep patterns, concentration difficulties and poorer memory performance, and transient increases in anxiety and depressed mood have been identified in healthy men with elicited immune activation by injection of endotoxin or low-dose administration of interleukin-6 (IL-6) in comparison with a placebo group.
8,
9 Cognitive impairments in attention, learning, and memory were associated with abnormal levels of IL-6 in individuals with lupus erythematosus, even after controlling for potentially confounding factors like depression and corticosteroid treatment.
10 Elevated serum levels of various immune mediators (TNF-alpha and receptors, neopterin, and IL-6) have also been associated with fatigue in patients with cancer,
11 sleep apnea,
12 acute coronary disease and recovery from surgery,
13 fibromyalgia,
14 and chronic fatigue syndrome
15,
16 when compared with patients or healthy controls without fatigue. As well, some studies have identified that individuals with major depressive disorder, with or without comorbid immunological conditions like multiple sclerosis and rheumatoid arthritis, may exhibit moderately elevated inflammatory responses, seen as elevations in neopterin, IL-6 and TNF-alpha, in comparison to nondepressed healthy or patient controls,
17 –
22 though studies have generally failed to find a direct correlation between severity of depression and the extent of elevation in biological markers.
19,
20 Moreover, some antidepressants have shown immunosuppressive responses both in vivo and in vitro.
23 Patients with treatment-resistant depression, however, may show a contrary response seen as an increased production of IL-6 to stimulation of immune cells in vitro when antidepressants were added.
24 Successful treatment with antidepressants has been linked to lower IL-6 pretreatment levels and decreased production of TNF-alpha posttreatment as compared with nonresponders or individuals with persistent depressive symptoms who had higher IL-6 production before treatment and no change in TNF-alpha levels with treatment.
25This exploratory study aimed to integrate biological studies on the neuropathogenesis of HIV within a broader clinical context by investigating the potential associations between systemic biological indicators of immune activation (i.e., TNF-alpha, IL-6, and neopterin levels) with the presence of specific common clinical symptoms in adults with HIV/AIDS: cognitive impairments (as measured by neuropsychological test performance) and subjective self-report of cognitive symptoms, fatigue, and depression. Based on the review of research in other medical conditions, we hypothesize that higher levels of biological measures will be associated with poorer performance in attention and memory and higher self-reported cognitive complaints, depressive symptoms, and fatigue.
METHODS
Participants
Thirty-one adults with confirmed HIV infection and no past or current use of highly active antiretroviral therapy (HAART) took part in this study. Given that antiretroviral medications are intended to alter viral and immune functioning, HAART-naive participants were selected to reduce the possibility that the variability in biological measures may be confounded by medications or other infections that accompany disease progression. This study was approved by hospital and university research ethics boards, and all participants provided written informed consent prior to their involvement. Interested individuals were screened to exclude those with preexisting neurological or developmental conditions (e.g., CNS opportunistic infection, seizure disorder, diagnosed learning disability, or head injury with loss of consciousness exceeding 30 minutes), lower than grade 6 reading level, significant medical illness (e.g., recent heart attack, kidney disease, or liver failure), history of psychotic disorder, current intravenous drug use or treatment for substance abuse, or taking medication (aside from antidepressants) known to significantly alter adrenocortical or immune function (e.g., prednisone, dexamethasone, fludrocortisone acetate, or cyclophosphamide).
The sample was comprised of 26 men (84%) and five women (16%) with mean age of 35.6 years (SD=7.9) and mean education of 13.4 years (SD=2.6). Participants were predominantly Caucasian (77%), and the majority reported sexual contact (90%) as a major risk factor for HIV infection. According to Centers for Disease Control and Prevention (CDC, 1992) disease stages classification, 18 were asymptomatic (58%: CDC A1 or A2), nine were mildly symptomatic (29%: CDC B1 or B2), and four had AIDS-defining illnesses or a nadir CD4 count of less than 200 (13%: CDC A3, B3, or C1-3). As a group, participants demonstrated mildly elevated symptom measures and mildly impaired neuropsychological test performance.
Measures
All data were collected on the same day with blood samples drawn in the early afternoon by trained personnel at the HIV Specialty Clinic and immediately delivered to participating laboratories according to standard safety guidelines. Participants were asked to abstain from alcohol at least the day before and to avoid eating any food or drinking caffeinated beverages at least 1.5 hours beforehand.
A general interview and the following questionnaires were administered:
•
Chelune’s Patient’s Assessment of Own Functioning Inventory (PAOF Total): subjective cognitive complaints;
•
Beck Depression Inventory (BDI Total or Cog-Aff): presence and severity of depressive symptoms; and
•
Piper Fatigue Scale—Revised (PFS-R Total): presence and severity of fatigue
The neuropsychological test battery was selected to assess domains commonly affected in individuals with HIV-associated cognitive impairment:
•
Attention/working memory: digit span and spatial span scores from the Wechsler Memory Scale—Third Edition;
•
Psychomotor efficiency and processing speed: Reitan’s Trail Making test parts A and B, Grooved Pegboard Test, and Smith’s Symbol Digit Modalities Test; and
•
Learning efficiency: Parker’s Repeatable Episodic Memory Test total scores on trials 1 to 3 and Ruff-Light Trail Learning Test total scores on trials 2 to completion.
Performance was summarized into composite clinical deficit ratings for the three domains and a grand total based on a modified application of Heaton’s Global Deficit Score. The validity of the Global Deficit Score has been demonstrated in adults with HIV infection and described as a better measure than individual test scores, especially for populations with “spotty” or subtle cognitive impairments.
26Estimates of TNF-alpha and IL-6 mRNA expression were obtained by real-time quantitative polymerase chain reactions procedures using SuperScript II reverse transcriptase (Invitrogen), Alta.I Prism 7900HT Sequence Detection System and SYBR Green detection with SDS version 2.1 software (Applied Biosystems). Each reaction was run in triplicate with results representing the average estimated copies of mRNA for each participant. Serum concentrations of IL-6, TNF-alpha, and neopterin were obtained using commercial enzyme-linked immunosorbant assay (ELISA) kits by trained laboratory technicians according to manufacturer guidelines (BioSource International & IBL Hamburg).
Data Analyses
One-tailed analyses with statistical significance defined as p<0.05 were used because of the directionality inherent in the hypotheses. Graphical and statistical diagnostics were employed to detect the presence of univariate or multivariate outliers, significant deviations from normality, nonlinearity, or heteroscedasticity.
27 The neuropsychological deficit scores and some of the biological assays (TNF-alpha mRNA, IL-6 Elisa, and neopterin) were positively skewed with many individuals’ scores falling within the normal range. Nonparametric tests or distribution-free tests were performed, as the application of common transformations to these variables did not significantly alter the distribution and could affect clinical interpretation.
27RESULTS
Spearman correlations for the biological indicators with PAOF total scores or with neuropsychological performance total and domain specific scores were nonsignificant (p>0.05). Higher BDI Cog-Aff scores and PFS-R total scores were modestly associated with elevated IL-6 mRNA expression (r s =0.40 and r s =0.38, p<0.05), and elevated serum neopterin was associated with higher BDI Cog-Aff scores (r s =0.28, p<0.05). On the other hand, Spearman correlations for TNF-alpha serum and mRNA expression with BDI Cog-Aff or PFS-R scores were nonsignificant (p>0.05).
Given the high intercorrelations between symptom measures, partial correlations were used to tease apart the relations with IL-6 mRNA expression. When the influence of depression was controlled, IL-6 mRNA expression was no longer correlated with PFS-R total fatigue (
pr =0.19, p=0.174). Conversely, the association between BDI Cog-Aff scores and IL-6 mRNA expression remained significant after removing the influences of subjective fatigue and cognitive complaints (
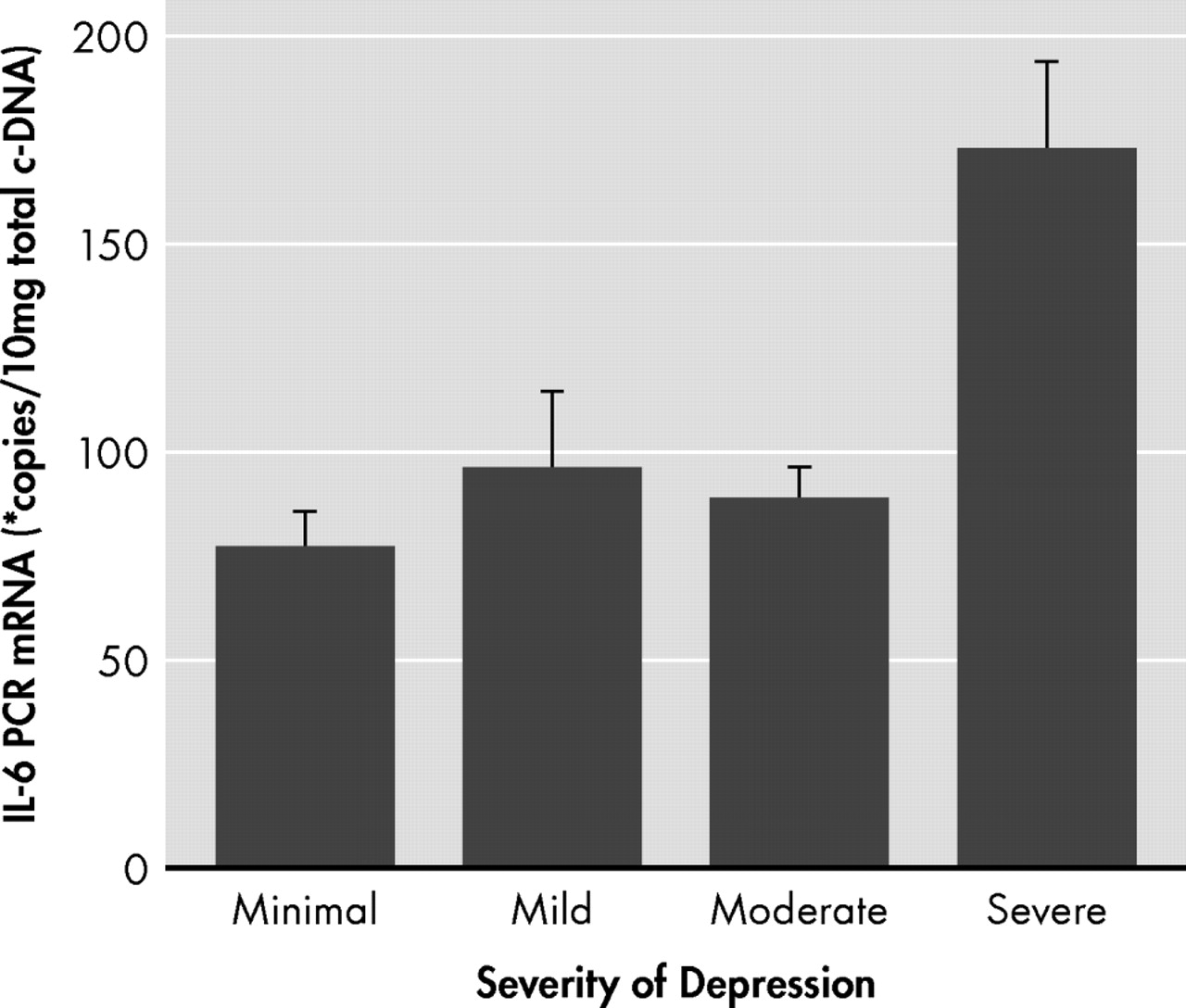
pr =0.39, p<0.05). One-way ANOVAs showed that differences existed between depression severity subgroups for IL-6 mRNA expression (F=7.72, df=3, 24, p<0.001). Bonferroni pairwise multiple comparisons showed that the severely depressed individuals had higher mean IL-6 mRNA expression compared with the means for the moderate (p<0.01), mild (p<0.01), and minimally (p<0.001) depressed groups (
Figure 1 ).
The influence of antidepressants on the association between biological indicators and depressive symptoms was explored by grouping participants into those currently using antidepressants (n=18) and those not taking any antidepressants (n=13). All types of antidepressants were considered together given the small sample size. No mean differences were evident between the individuals taking and not taking antidepressants on the demographic variables or symptom measures, with the exception of the mean age for the antidepressant group (μ=38.8, SD=9.0) being slightly higher than the mean age of those not taking any antidepressants (μ=33.2, SD=6.3) (F=4.33, df=1, 29, p<0.05). An interesting interaction was, however, observed for neopterin serum concentrations and depressive symptoms across antidepressant groups. Although neopterin and depressive symptoms were strongly associated in the antidepressant group (
r s =0.83, p<0.001;
r =0.78, p<0.001), the association was nullified in the group not taking antidepressants (
r s =−0.25, p>0.05;
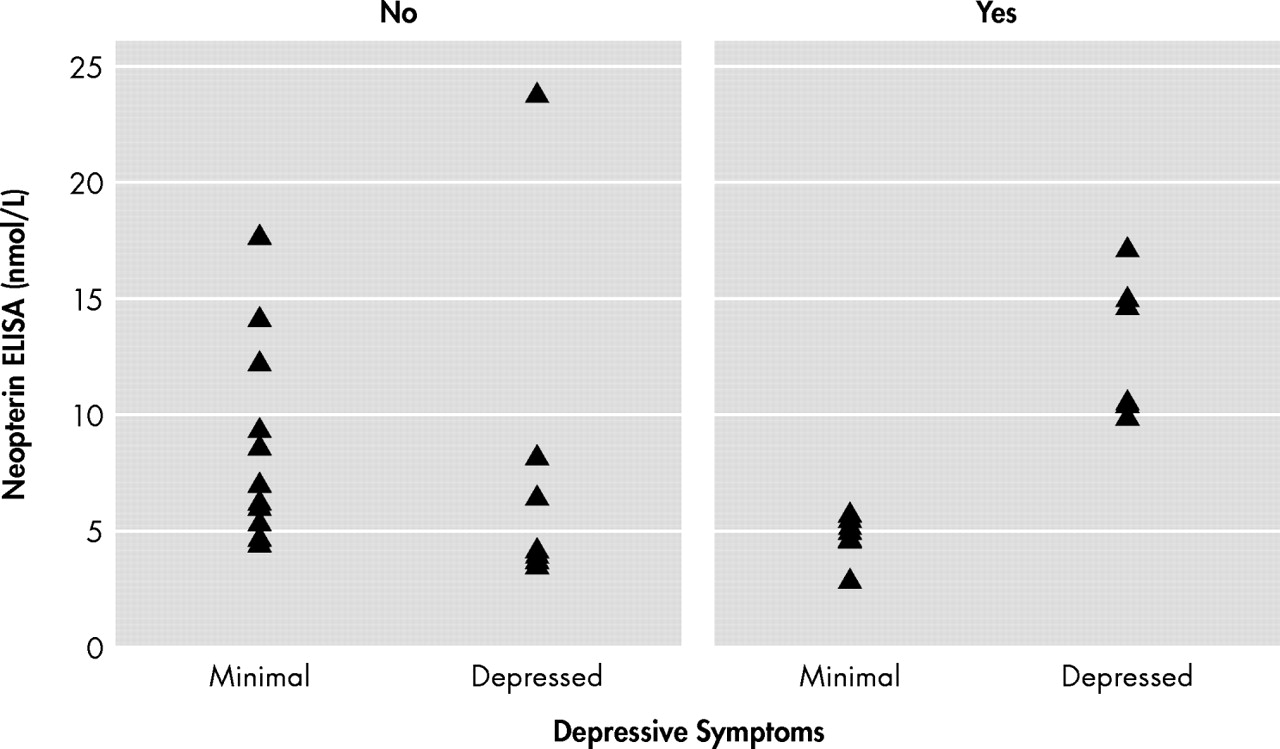
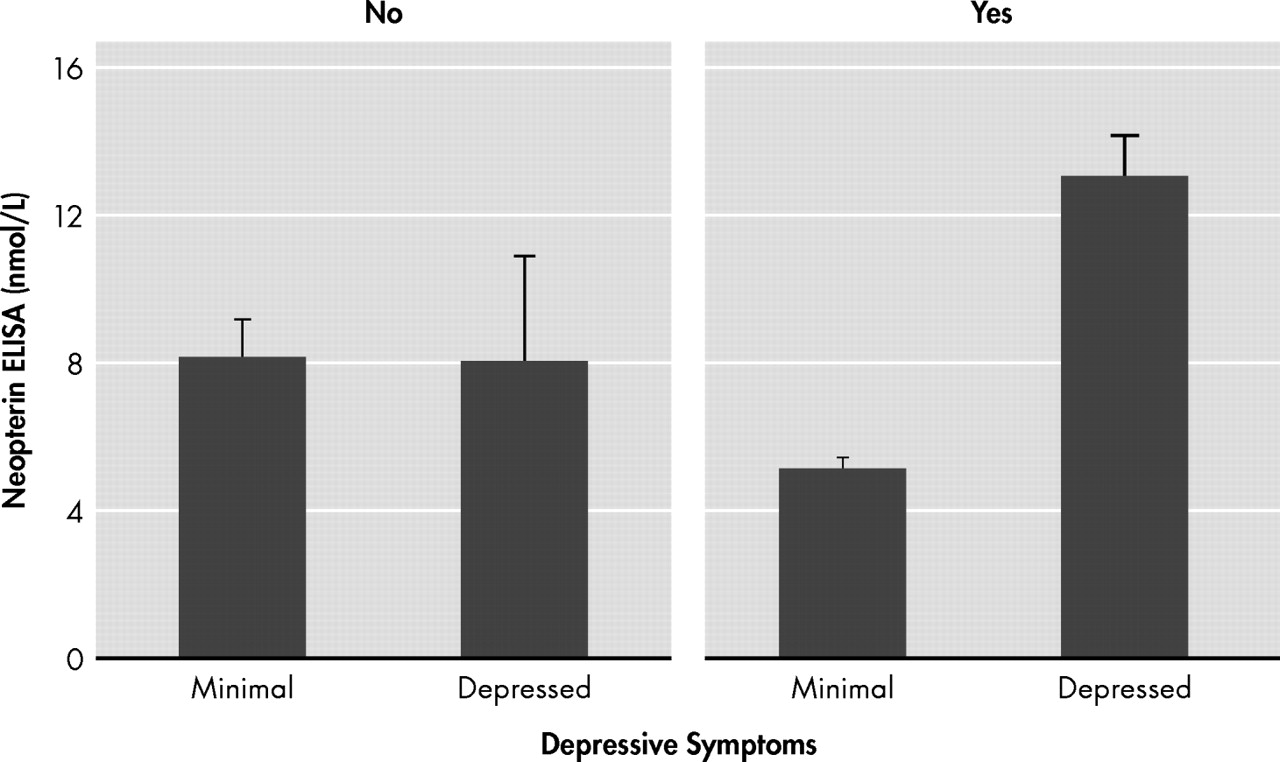
r =−0.08, p>0.05). In order to further investigate the nature of this interaction, individuals were divided into not depressed and depressed groups based on a BDI cognitive-affective cutoff score of 10. Participants’ concentrations of neopterin for depressed versus nondepressed individuals are separately shown by antidepressant groups in
Figure 2 . Depressed individuals taking antidepressants (n=6) showed higher neopterin serum concentrations in comparison with nondepressed individuals taking antidepressants (n=7). In fact, all six depressed individuals on antidepressants were above the clinical reference range for neopterin (i.e., cutoff>10 nmol/liter) while all seven nondepressed individuals on antidepressants were within normal expectations. More variability was seen among both the depressed and nondepressed individuals not on antidepressants, though most neopterin levels were within normal limits. ANOVAs separately performed by antidepressant group provided further support for an interaction between depression, antidepressants, and neopterin levels. Although mean neopterin levels of the depressed and nondepressed groups were not different for those not taking antidepressants (F=0.00, df=1, 15, p>0.05), mean neopterin levels were higher in the depressed as compared to nondepressed group for those taking antidepressants (F=45.66, df=1, 11, p<0.001) (
Figure 3 ).
DISCUSSION
The major findings were as follows: (a) neuropsychological impairment and subjective cognitive symptoms were not associated with serum biological measures of immune activation; (b) presence and severity of depressive symptoms were related to some measures of immune activation (i.e., elevated neopterin and mRNA expression of IL-6); and (c) the association between immune activation and depressive symptoms was distinct in those taking and not taking antidepressants.
Physicians typically initiate HAART treatment when CD4 T cell counts significantly drop and symptoms ensue; thus the majority of this HAART-naive sample was asymptomatic (58%) or mildly symptomatic (21%). Cognitive impairments tend to increase in prevalence with duration of the illness and advancing disease stage.
6,
7 Research on the cellular mechanisms underlying neurotoxicity suggests that the HIV virus does not directly infect neurons but instead is stored and transported to the brain via macrophages. The release of viral and cellular toxins (e.g., proinflammatory cytokines or oxygen radicals) from chronic low-grade activation of macrophages is thought to indirectly damage the CNS.
4 Given the complexity and resilience of the brain, multiple insults exacerbated by transient states of heightened immune activation (that also perpetuate phases of more intense viral replication) may be required prior to producing significant cognitive impairments as measured by below expected performance on neuropsychological tests. Neuropsychological testing provides an estimate of the individual’s best cognitive performance within an isolated, time-limited context. However, this performance may be unrepresentative of the ongoing demands faced in everyday situations especially in individuals with relatively mild changes in their cognitive abilities. While transient fluctuations in proinflammatory cytokines and immune activation may contribute to the mild, intermittent cognitive inefficiency experienced in everyday life, a single session of objective neuropsychological tests may not capture subtle early cognitive changes. Thus, the restricted variability in neuropsychological test performance (i.e., most scores within normal limits) in individuals at early disease stages and single time point of analysis may have contributed to the limited findings in the present study, as compared with previous investigations that have shown peripheral immune responses to be linked with cognitive dysfunction at advanced stages.
6,
7While the sample size is adequate to detect a moderate or strong correlation, a much larger sample size (N>150) may be needed to detect a more modest association.
27 Although some preliminary studies with similar sample sizes have obtained modest correlations between biological indicators and cognitive impairments in other medical conditions or in healthy individuals under experimental manipulations,
8 –
10 larger samples of adults with HIV infection or reactive paradigms may be needed given the demographic and clinical variability in this population. For example, other studies have required large sample sizes in order to capture small but clinically significant relations between neuropsychological performance and subjective cognitive complaints in samples of adults with HIV infection.
28,
29In the present study, elevated immune activation (as measured by serum neopterin levels and mRNA expression of the proinflammatory cytokine IL-6) was modestly associated with depressive symptoms. Higher serum IL-6 mRNA expression was also modestly associated with elevated fatigue, though this relation was weakened when the overlapping influence of depression was removed. More specifically, both the
presence and severity of depressive symptoms were related to elevated immune activation, such that the most severely depressed individuals in this sample showed higher levels of IL-6 mRNA expression and neopterin concentrations. Similarly, several recent investigations have shown heightened immune activation, as measured by both elevated serum IL-6 and neopterin, in depressed individuals as compared to nondepressed controls,
17 –
20,
22 though the association between biological measures and the severity of depression has been less consistently found.
19,
20 While condition/control group differences are initially useful in identifying measures of interest to study, they provide limited information about the individual variability in symptom expression within a medical condition. More in-depth exploration of factors within a specific condition is necessary for identifying subgroups and associated characteristics that may place particular individuals at higher risk for the development of certain clinical symptoms.
In this study, the influence of antidepressants’ potential immunosuppressive effects on the biological indicators was explored. Neopterin and depressive symptoms were strongly associated in the group of individuals currently taking antidepressants, but not in individuals without antidepressant treatment. In the antidepressant group, some of the individuals showed lower neopterin levels and reduced depressive symptoms, which would be consistent with expectations if antidepressant medication was effective. In contrast, a subsample of individuals on antidepressants continued to endorse high levels of depressive symptoms and had elevated neopterin levels. This subsample may represent individuals not responding to the treatment similar to previously described “treatment-resistant” groups.
24,
25While these preliminary findings require replication in a larger sample, they highlight the importance of considering multiple etiological factors when interpreting and managing clinical symptoms on an individual basis. Although the exact mechanisms underlying these associations cannot be derived from the current analyses, this study provides support for the role of biological processes (either on their own or as mediators of psychological factors) in the differential expression of clinical symptoms in HIV. More specifically, this study supported previous work
25 that measured immune activation markers after the attainment of therapeutic drug concentrations as a potential means to differentiate depressed individuals who are less likely to respond to antidepressant medications and consequently are at greater risk of developing chronic depression. In addition to IL-6, neopterin may be a good candidate to explore further as a more global indicator of immune activation, especially macrophage activity, which is of interest in the underlying HIV mechanism. If validated, a biological screen for early identification of potential patients less responsive or resistant to antidepressants (e.g., individuals with elevated neopterin levels and persistent depressive symptoms after just a few weeks of treatment) may allow clinicians to efficiently implement alternative effective interventions (e.g., stress management techniques/psychotherapy alone or in combination with drug therapies) aimed at alleviating the depressive symptoms of these individuals and improving quality of life. Examination of biological factors pre- and postinterventions may assist in monitoring the progress of susceptible individuals and may provide a better understanding of the characteristics that affect differential responses to antidepressant treatment. Prospective research designs that examine the progression of symptoms and monitor biological changes at regular time intervals may be particularly fruitful in tracking the pattern of associations. To date, studies on antidepressant effects (including the present investigation) have been limited by confounding factors. Future research should attempt to control for the type of antidepressant (i.e., examining the effects of one antidepressant rather than a mixture of antidepressants) and the duration of treatment. As well, individual differences rather than group means may provide more insight into the characteristics of this subsample. During enrollment in such a study, it will be important to define and carefully monitor potential biological changes in response to naturally occurring stressful transition points during the course of HIV (e.g., diagnosis of HIV seropositive status, initiation of HAART, or diagnosis of AIDS) or in response to unpredictable life stressors (e.g., job loss, break up of a close relationship, or death of a family member). The collection of multiple samples over the course of the day or in response to experimental stimulation of the immune/HPA systems (e.g., lipopolysaccharide challenge, exposure to psychological stressors, or under dexamethasone suppression) may prove to be more sensitive measures of biological processes than the isolated serum samples used in the present study.
Acknowledgments
This study was supported by grants from the Canadian Foundation for AIDS Research (CANFAR) and the Ontario HIV Treatment Network (OHTN) to Sean B. Rourke and an OHTN Studentship to Erin M. Warriner.