HIV-associated neurocognitive disorders (HAND) remain important even in the era of highly active antiretroviral therapy (HAART). Although a decline has been noted in the incidence of HIV-associated dementia (HAD) in the developed world since the introduction of HAART, HIV-associated neurocognitive disorders (HAND) remain prevalent.
1 Despite the relative efficacy of HAART in controlling HIV disease, there is no treatment that specifically targets the cause of HAD nor which prevents neuronal damage caused by the virus. Although first recognized two decades ago, as a subacute, subcortical dementia, the clinical syndrome has evolved, in part due to the use of antiretroviral drugs.
2 In South Africa, the roll-out of HAART has not reached levels seen in the developed world.
Early studies suggested that individuals infected with clade C may be less likely to develop cognitive impairments due to a biological defect in the tat protein present in clade C.
3 However, in recently published data from India, it was shown that individuals infected with clade C exhibit significant cognitive impairment, suggesting that the brain is, in fact, vulnerable in individuals with clade C HIV.
4These findings are notable because clade C is the most common form of HIV in the world, as well as the dominant strain in South Africa.
3 In support of this finding, a study using the HIV Dementia Scale (HDS) reported HAND in 23 patients, 5% of patients attending HIV clinics in South Africa.
5Although much work has been done to elucidate the complex mechanisms underlying HIV-associated neurotoxicity, the relationship between cognitive impairment and white-matter integrity in HIV remains incompletely understood. This is particularly the case for the genetic form of HIV known as clade C, since nearly all studies of cognitive function in HIV have been conducted in North America, where the dominant genetic strain of the virus is clade B. Autopsy studies of HAD patients indicate prominent injury, including damage to the deep white matter.
6 A range of neuroimaging modalities have been used to study the relationship between white-matter damage and neuropsychological impairment, which has identified subcortical gray and frontal white matter as the principal sites of early abnormalities.
7 HAD is associated with diffuse atrophy, with ventricular dilatation
8 and white-matter lesions. A correlation between impaired cognitive function and the loss of volume in certain brain structures, including the basal ganglia and caudate nucleus, has also been reported.
9 These findings are consistent with the characteristics of the early neurological deficits, and the known predilection of HIV for the basal ganglia.
10There are limitations to these methods. In HIV infection, white-matter changes are unreliably detected by structural MRI (sMRI). Fortunately, diffusion tensor imaging (DTI) is able to provide information about the micro-structural integrity of the white matter. The most commonly used DTI-derived metrics are fractional anisotropy (FA), which reflects the orientation specificity of water diffusion, and mean diffusivity (MD), which reflects the degree of water diffusion within an imaging voxel.
11 Decreased FA and increased MD often reflect chronic white-matter change. DTI studies in HIV have shown diffuse damage to cerebral white matter (i.e., decreased FA or increased MD), and these effects have been found to be particularly prominent in the advanced stages of the disease. Studies conducted in countries where clade B is predominant have reported subcortical white-matter injury mainly in the regions of the corpus callosum, frontal lobes, and internal capsule.
12–14 Furthermore, diffusion abnormalities in the corpus callosum in HIV patients have been associated with dementia severity and motor speed losses.
12The present study examined the relationship of DTI indices to global neurocognitive impairment among HAART-naive patients infected with clade C HIV. We hypothesized 1) that Clade C HIV infection would be associated with reduced white-matter integrity in the regions of the corpus callosum, frontal lobes, and internal capsule; 2) that more white-matter injury would be evident among individuals with HAD.
METHOD
Subjects
Participants (N=46) with HIV and healthy non-HIV controls (N=10), age 18–35 years were recruited from the Infectious Diseases Clinics within the Groote Schuur Hospital recruitment area. Controls tested as being HIV-negative attended the same ARV community clinic as the subjects, thus potentially controlling for confounding variables such as socioeconomic status and risk-taking behavior. To be eligible, HIV participants were required to have a positive diagnosis of HIV infection made within the last 6 months (based on serologic testing by ELISA and Western blot), and have not previously used antiretroviral medications (“HAART-naive”) Also, patients were required to have at least 7 years of formal education. Participants in the study were all African. Hepatitis sero-status was not established, but the prevalence of hepatitis C in South Africa is extremely low.
15Patients were excluded if they were 1) using psychotropic medication; 2) had a recent (6 month) substance abuse history as defined by responses to the Alcohol Use Disorders Inventory (AUDIT) and the Substance Abuse and Mental Illness Screener (SAMISS); 3) had a score greater than 16 on the Beck Depression Inventory (II); 4) had other current DSM-IV disorders as determined by the MINI; or 5) had a lifetime history of head injury with loss of consciousness.
Procedure
Participants completed clinical assessments in a single testing session. Neuroimaging was conducted within 1 week of the initial evaluation. All measures were administered and scored according to standard procedures. Written informed consent was obtained before enrollment and exposure to any study-related procedure. The protocol was approved by the Committee for Human Research of the University of Cape Town.
A neuropsychological test battery (
Table 1) was administered to all participants to assess specific domains of attention, concentration, learning, memory, psychomotor speed, and executive function. The battery was based on that used by the HIV Neurobehavioral Research Centre,
1 and included tests were adapted in discussion with three local neuropsychologists. Changes to word lists to reflect local language and idiom were made. All instruments had their instructions and content translated into isiXhosa and Afrikaans; instructions were also back-translated for fidelity. A full neuromedical examination was conducted on all participants. Blood for CD4 cell counts was taken in the infectious diseases clinic.
Determination of Dementia
The neuropsychological test battery, together with scores from a neuromedical assessment and reported functional assessment was used to classify participants into one of four HAND categories, based on the updated American Academy of Neurology criteria:
1 no impairment; asymptomatic neuropsychological impairment (ANI); mild neurocognitive disorder (MND); and HIV-associated dementia (HAD). Using the modified AAN classification, participants who scored >2 standard deviations (SD) below the z-score cut-offs on at least two domains of functioning were rated NP2; those who scored between 1.0 and 2 SD below on two domains of functioning, or >2 and 1–2 SD below on two domains were rated NP1. In order to allow for comparability with other studies, we also categorized participants into the corresponding Memorial Sloan Kettering (MSK) scores. An MSK severity score of 0 represents no cognitive impairment; 0.5 represents mild cognitive impairment not sufficient to meet criteria for dementia; and a score of 1.0 represents dementia.
16 The final classification was conducted by a consensus panel comprising two HIV neuropsychiatrists (JJ, JH) and an experienced neurologist (MC). Data from 51 HIV-negative control subjects were used to generate z-scores for establishing local norms.
Brain Imaging
Imaging was performed on a Siemens Magnetom 3T Allegra within 7 days of the screening and neuropsychological assessment. We acquired a multidirectional diffusion weighted sequence with 30 diffusion directions, 1 b=0 sec/mm2 direction, TR=8,800 msec, TE=88 msec and a b-value=1,000 sec/mm2. The images were acquired as a mosaic, yield a 960 × 960 matrix with 60 slices per volume, and a corresponding in-plane spatial resolution and slice thickness of 2 × 2 mm2 and 2.2 mm, respectively. The 2.30-minute sequence was repeated three times, and averages were derived.
Image-Processing
Eddy-current correction was performed separately in each of the three acquisitions, using affine transformations in FSL (Oxford, England, Center for MRI of the Brain; Oxford, England). The data were then imported to MATLAB (The Mathworks Inc, Natick, MA) for further processing. Co-registration was performed across the three acquisitions, using affine transformations with the unweighted image of the first average as a reference. For each of the three acquisitions, diffusion tensors were calculated and outliers were rejected by first calculating z-scores based on 25- and 75-percentile limits, and then discarding data-points more than 3 SD away from the mean. The three acquisitions were then averaged, and mean diffusivity (MD) maps were calculated. Diffusion tensors and fractional anisotropy (FA) images were then derived. An affine as well as a nonlinear registration of the B0 images to each subject's structural T1 image (intrasubject) was performed. The warps were applied to the FA images. A mean FA template was then created from all the subjects, followed by a final registration and warp of all the FA images to the mean template. The same registration-transforms were applied to the MD images. A general linear model ANOVA was performed on the FA and MD voxel of each cohort to determine significant differences between the groups. This was performed at a p<0.01 uncorrected threshold with an FA mask of 0.25. A whole-brain voxel analysis of FA and MD was performed for each cohort.
Statistical Analysis
Multivariate tests of overall differences between HIV-positive groups and the healthy sero-negative control (HC) group were conducted for motor function, learning and memory, attention, speed of processing, or executive function domains. Also, three linear-regression models were created to determine the independent associations between the individual neuropsychiatric tests and FA values in the regions of the CC rostrum, sagittal stratum, and the cingulum. Because of sample size limitations, if high correlations existed between the individual psychiatric tests in each domain (
Table 1), only one was included in the model. Also, since there were significant differences between cases and controls in gender, age, and years of education completed (
Table 2), these variables were controlled for in all analyses.
Contrasts between HIV-positive groups and the HC group were then drawn to examine the associations between HIV infection, cognitive impairment, and white-matter FA. Whole-brain voxel analysis of FA and MD in the HC group was compared with the whole HIV+ cohort, as well as the subgroups of HIV+ (no cognitive impairment [NAD]), mild cognitive impairment (MND), and dementia (HAD). Finally, the subjects with HAD were compared with the rest of the HIV+ cohort.
RESULTS
Of the 56 subjects originally enrolled, 54 participants (HIV+: N=44; HC: N=10) were included in the final analysis. The imaging data from two subjects in the HIV group were discarded because of movement artifacts. The average age of the HIV+ and HC participants was 29 and 26 years, respectively. The average level of education for the HC cohort (11.56 years) was higher than for the HIV cohort (9.63 years). The mean CD4 count of the HIV+ group was 211 (SD: 141). The percentage of participants in each group differed significantly by gender (p=0.049), age (p=0.017), and education (p=0.007).
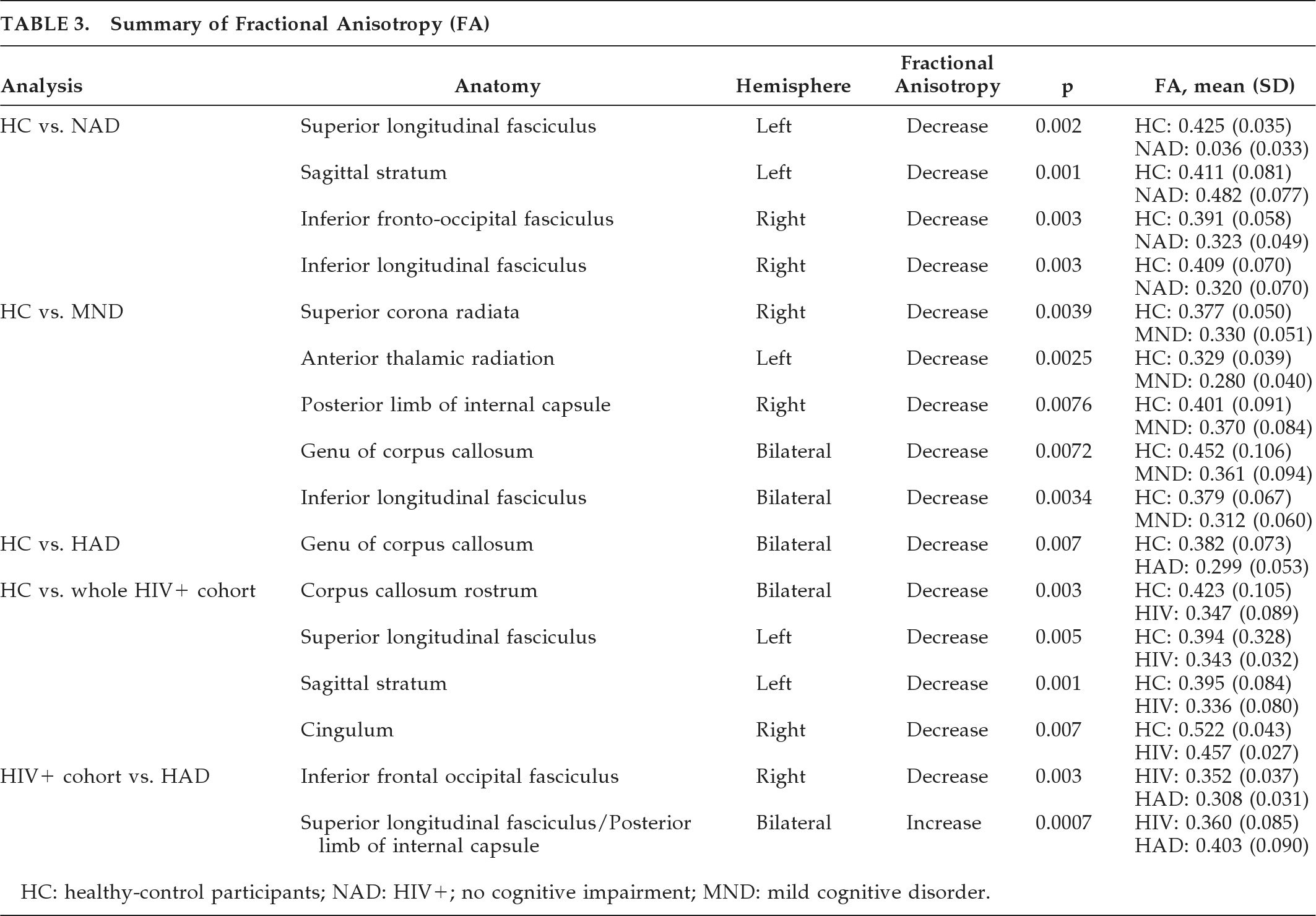
Diffusion Tensor Imaging
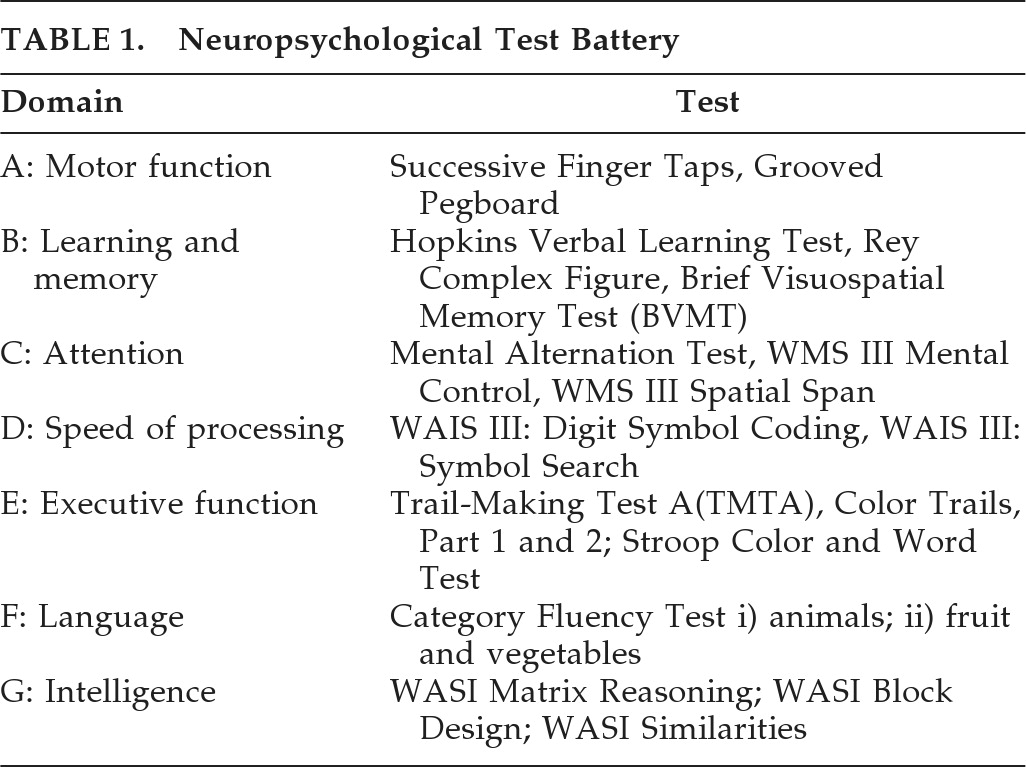
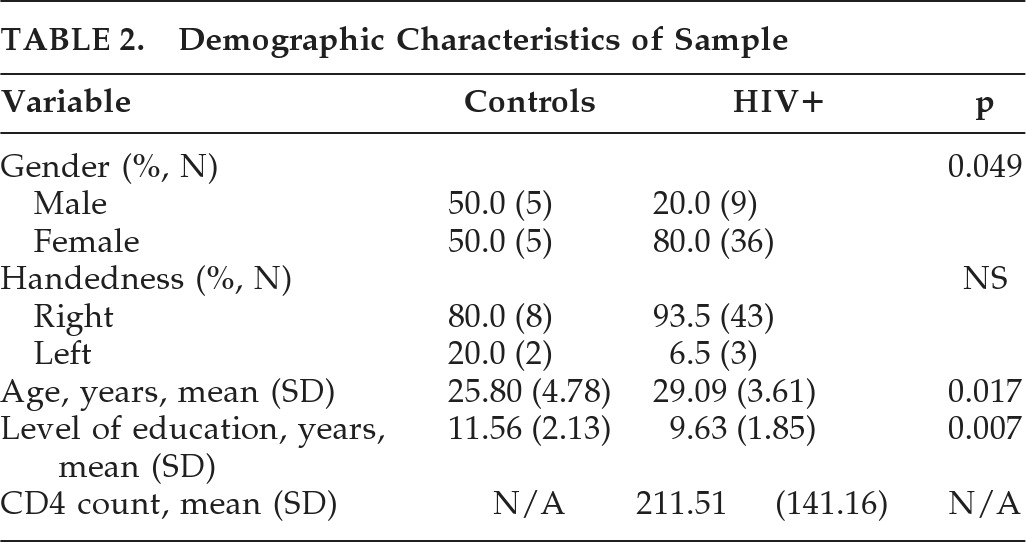
Individual FA values were found to be significantly lower in HIV+ patients in the corpus callosum (CC) rostrum (p<0.001;
Figure 1), the sagittal stratum (p<0.001;
Figure 2), and the cingulum (p=0.003), as compared with HCs). Other comparisons to the HCs were made, using a NAD group (N=4), a group with MND (N=26), and a HAD group (N=14). A final comparison was made between those with HAD and the rest of the HIV+ cohort. The FA results are summarized in
Table 3.
The multivariate tests of overall differences among groups were not statistically significant for the motor function, learning and memory, attention, speed of processing, or executive function domains, when controlling for gender, age, and years of education completed.
Three linear-regression models were created to determine the independent associations between the individual neuropsychiatric tests and FA values in the regions of the CC rostrum, sagittal stratum, and the cingulum. Although the overall model predicting FA values in the CC rostrum was not significant, WMS III Mental Control was a significant predictor (b = –0.75; t = –3.21; p=0.004). However, the overall model predicting FA values in the sagittal stratum region was statistically significant (F [19, 26]=2.08; p<0.041), with WMS III Mental Control remaining as the only significant predictor (b = –0.61; t = –2.88; p=0.008). None of the individual psychiatric tests significantly predicted FA values in the cingulum region.
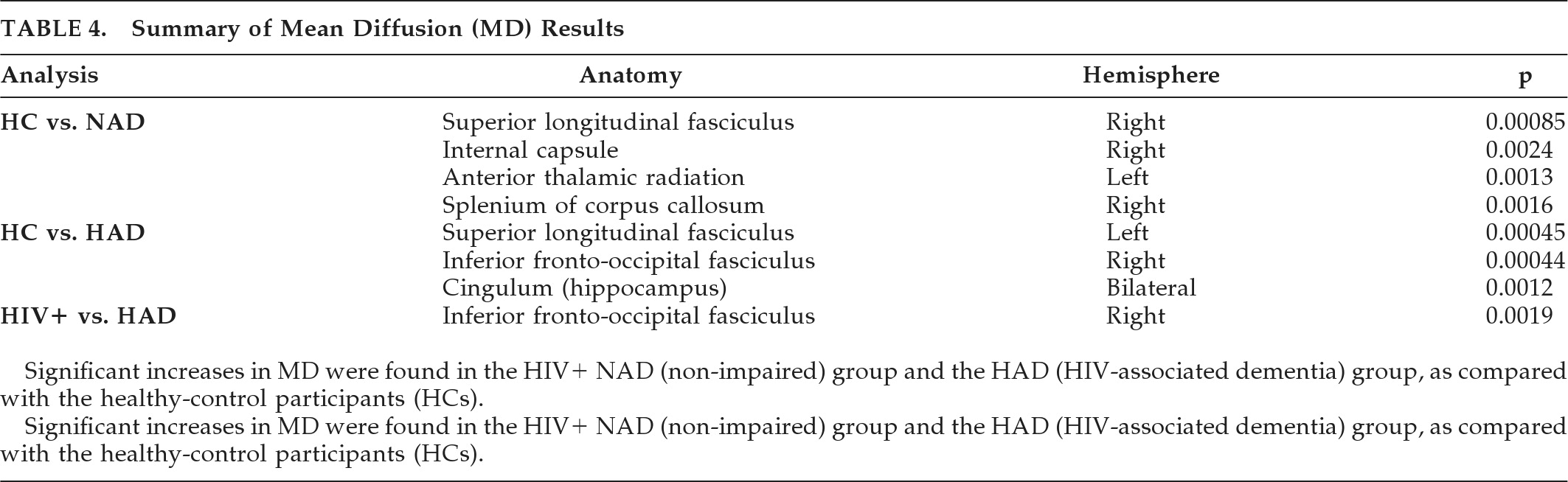
The same group comparisons were made measuring MD (
Table 4). No significant differences were found between HC and HIV+ participants, or between HCs and those with mild cognitive impairment. However, significant increases in MD were found in the HIV+ NAD group and the HAD group when compared with the HCs. This may suggest that inflammation is greatest in the very early and late stages of HAND (a biphasic inflammatory model). When comparing patients with HAD to the rest of the HIV+ cohort, MD was increased in the inferior fronto-occipital fasciculus.
DISCUSSION
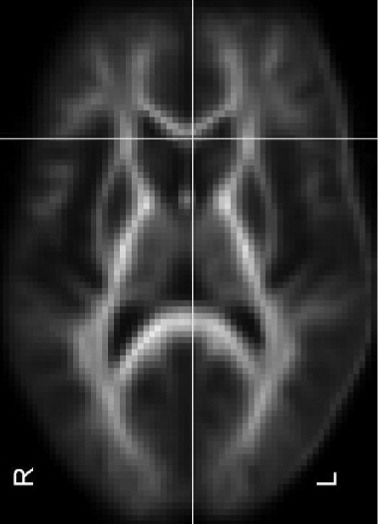
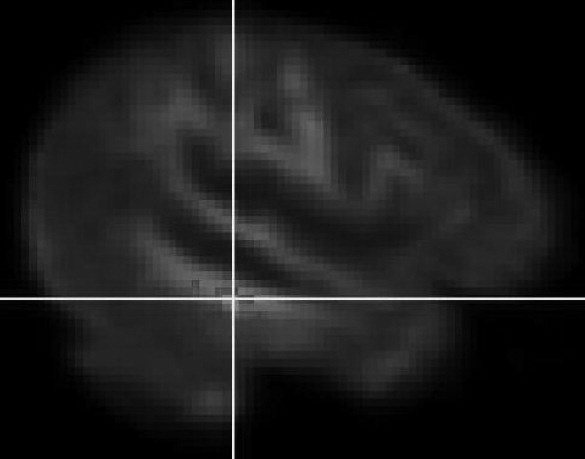
This study confirms that DTI-detected micro-structural white-matter abnormalities are present in clade C HIV when images are compared with those of healthy controls. These changes were more extensive in those with HIV-associated dementia. These include MD and FA changes in the inferior fronto-occipital fasciculus. We were able to detect white-matter abnormalities in HIV+ cognitively normal individuals and HIV+ participants with mild cognitive impairment, suggesting loss to white-matter integrity early in the disease process. Although the overall model of neuropsychological tests predicted lower FA in the sagittal stratum, there were few associations between individual neuropsychological tests and lower FA in the regions identified. Increased MD detected in the cognitively normal (NAD) group (
Figure 3) and the HAD groups only, suggests a biphasic inflammatory process in HIV dementia.
Our study indicates that, in keeping with previous work in clade B, individuals infected with clade C HIV have white-matter abnormalities. The white-matter damage has previously been found to be more prevalent and severe in patients with HIV-1–associated dementia.
17 Consistent with a previous DTI studies, we demonstrated preferential injury to the corona radiate, corpus callosum, and superior and inferior longitudinal fasciculus.
18 Involvement of the corpus callosum in HIV encephalitis has been reported in post-mortem findings.
19 A number of DTI studies in HIV have reported white-matter damage to the corpus callosum.
12,13,20We found that HIV+ individuals without HAND also demonstrated white-matter changes. Thus, HIV-related neurotoxicity occurs in the absence of clinical features of cognitive impairment. DTI abnormalities have previously been reported in the FWM of cognitively asymptomatic HIV+ patients with normal structural MRI studies.
11We also found increases in FA and MD in the HIV dementia group, as compared with the rest of the HIV+ cohort in the superior longitudinal fasciculus. The standard interpretation of these results are that increased FA in the areas represent increased white-matter integrity in HAD participants compared to participants with MND. However, Stebbins et al.
21 found similar increases in FA and suggested that increased FA could be due to loss of the complexity of the white-matter matrix in those regions. If a voxel contains only parallel white-matter fibers, FA can be high. Damage to the matrix would result in loss of crossing, with sparing of the parallel fibers and a paradoxical increase in FA. One indicator of such a process would be an increased MD, which suggests general tissue damage, together with the increased FA.
20 This relationship is seen in the superior longitudinal fasciculus in this study.
WMS III Mental Control predicted reduced FA in the sagittal stratum and corpus callosum, suggesting that this neuropsychological test may be sensitive to white-matter damage in clade C HIV. It is possible that this may be a clade-specific difference, wherein we found that HIV+ participants performed as well as HIV-negative controls on the grooved pegboard test, a measure consistently used to ascertain whether HIV-associated subcortical neuropathology exists (Sacktor et al.,
22 Joska et al., 2009, unpublished). Previous DTI studies with clade B found correlations between motor-speed losses and poor white-matter integrity of the corpus callosum.
14Data from Alzheimer's disease research show that maximal immunoreactivity in neurons occurs during the early and very late disease stages. The increases in MD that we detected follow a similar biphasic pattern.
The neurocognitive impact of the clade C HIV has not been determined. A study in southern India among individuals with the clade C virus with advanced HIV disease suggested that cognitive difficulties are present among individuals with the clade C virus in India, with more than half of the patients with advanced HIV meeting the criteria for impairment in two cognitive domains.
4 However, investigation of the neuropathogenesis induced by HIV-1 clades using an animal model, revealed greater astrogliosis and increased loss of neuronal network integrity and an increased number of memory errors in clade B than in clade C.
24 Additional study is needed to determine whether clade C HIV infection is more or less likely to cause neurocognitive deficit than the clade B virus. Furthermore, the impact of antiretroviral therapy on neurocognitive dysfunction in clade C viral infection needs to be determined.
Limitations of our study include the relatively small samples, and the cross-sectional design. The data here are not able to address the question of whether white-matter changes noted in the NAD and MND groups represent an early marker of subsequent cognitive decline.
Despite these limitations, findings here revealed lower FA and increased MD in the HIV+ groups in the corpus callosum and other regions. Future work could usefully focus on anisotropy measurements as quantitative imaging biomarkers in the setting of HANDs, using larger, longitudinal studies. Identification of reliable early imaging correlates of risk for subsequent cognitive dysfunction, such as early changes to white-matter integrity, may allow for earlier treatment with HAART and delay the course of HIV disease. Studies examining response to HAART treatment in patients infected with HIV will be important to determine whether DTI abnormalities observed in the corpus callosum and other regions reflect reversible or more advanced, irreversible injury. Correlates of white-matter damage and neurocognitive decline need to be sought: whether measures of central viral load correlate with poor FA values, illness duration, age, treatment exposure, and treatment adherence. These factors are almost certainly critical in determining the overall impact of HIV on brain function, and, in particular, on white-matter integrity. In the interim, these findings point to the potential value of FA and MD as a measure of CNS damage in HIV+ patients.
Acknowledgments
Location of work: Department of Psychiatry and Mental Health, University of Cape Town, Cape Town, South Africa, and Cape Universities Brain Imaging Centre Faculty of Health Sciences, University of Stellenbosch, Tygerberg, 7505 South Africa.
JH has received support from the Medical Research Foundation of South Africa and the Discovery Foundation Academic Award of South Africa. JJ has received support from the National Research Foundation, the Biological Psychiatry special interest group of the South Africa Society of Psychiatrists, the Medical Research Foundation of South Africa, and the Faculty of Health Sciences Research Committee, University of Cape Town.