Several studies have drawn attention to the correlation between PSs and stroke. Depression and apathy have been reported as the most frequent disorders.
3–6 Few studies have focused on the occurrence of depression or other psychiatric disorders in the acute and early post-acute phase of stroke, which is a crucial period for patients' rehabilitative treatment.
7 Some authors have investigated the influence of premorbid personality traits on the occurrence of PSs after stroke,
4,8 but psychopathological status before the event and its predictive potential for poststroke PSs have not been studied.
Some factors occurring in the acute phase of stroke, such as low consciousness level, aphasia, or comorbid pathologies, can limit patients' abilities to manifest and communicate their PSs.
7 The Neuropsychiatric Inventory (NPI) uses a reliable informant to quantitatively assess a patient's behavioral symptoms over the past 4 weeks.
9 The NPI was designed for patients with dementia, but it has also been used in such neurological disorders as multiple sclerosis
10 and stroke.
5,6 Given that this test does not require the patient's collaboration, the NPI can be used to provide information about the patient's psychopathological status before, and in the weeks immediately after, the occurrence of the stroke.
The aim of this study was to identify predictive factors of PSs associated with ischemic stroke that could be detected at the onset of the stroke, with a special focus on premorbid psychopathology, in order to identify a patient profile at risk of developing poststroke PSs. We also evaluated the frequency and evolution of these PSs as well as their correlation with the patient's cognitive and functional status.
RESULTS
Subject Characteristics and Clinical Features
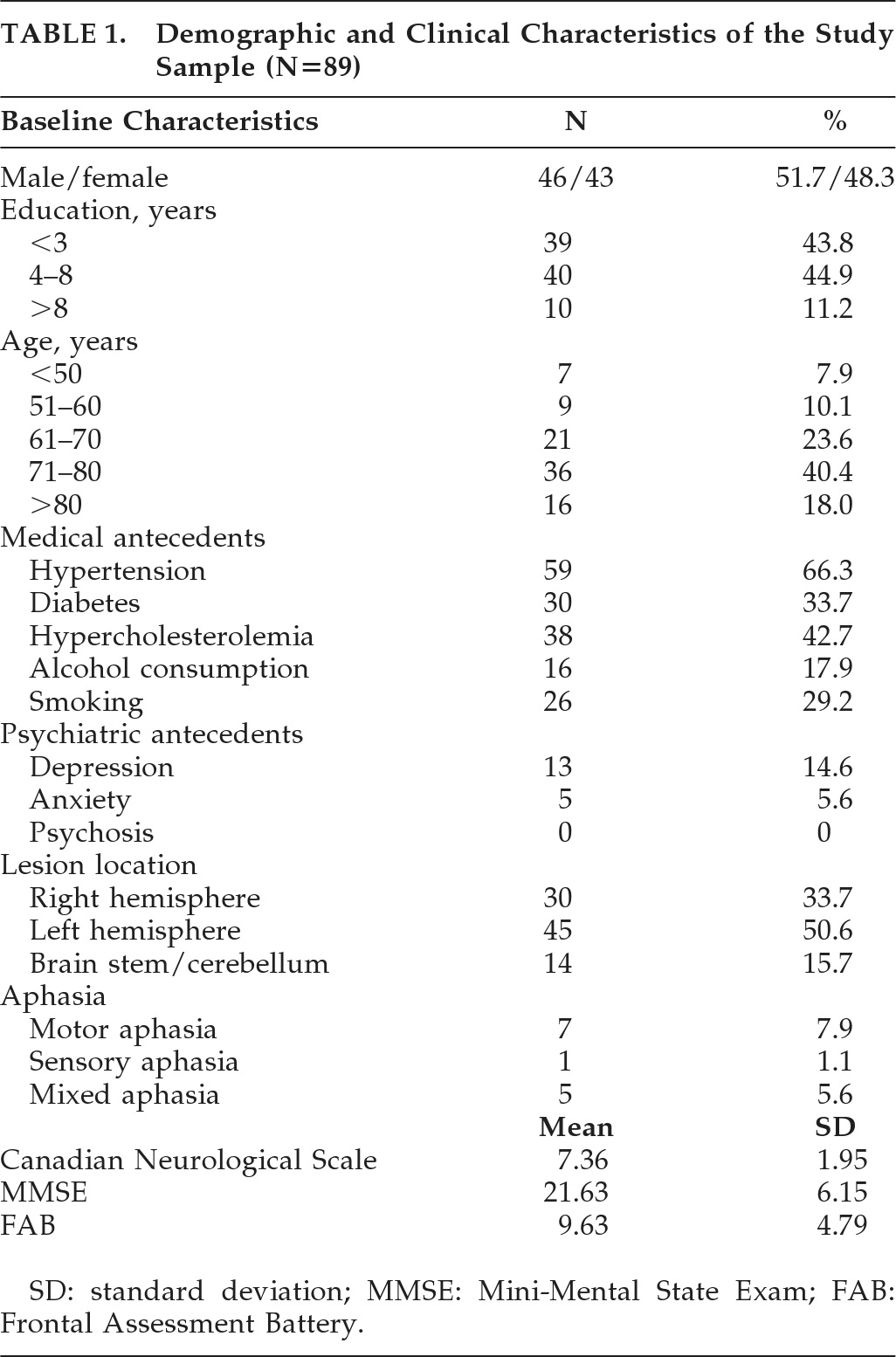
Eighty-nine patients fulfilled the inclusion criteria and met none of the exclusion criteria.
Table 1 details their baseline demographic data, medical and psychiatric antecedents, and clinical characteristics.
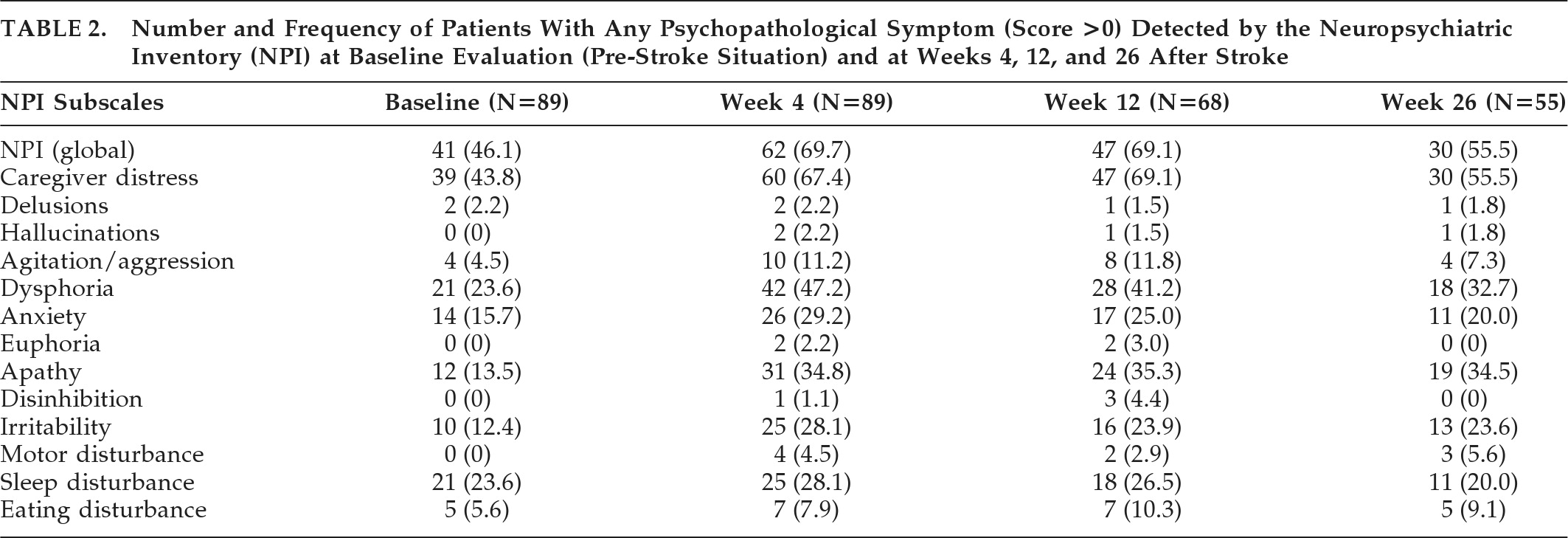
The number and percentage of patients who presented at least one PS, defined as a score >0 in any NPI subscale, at baseline (before stroke) and 4, 12, and 26 weeks after the acute event, are represented in
Table 2.
Eighty-three patients were able to accomplish an assessment with Hamilton's scales (Ham-D and Ham-A). Among the other six patients, five had a severe aphasic disorder, and the remaining patient refused to perform the test; 38% of patients showed detectable depression on the Ham-D (defined as a score >7). Moderate-to-severe depression (defined as a score >14) was seen in 19.9% of patients, whereas anxiety was detected with the Ham-A (defined as a score >5) in 33.7% of patients. Moderate-to-severe anxiety (defined as a score >14) was detected in 6% of patients. Pearson′s correlation coefficient between the NPI Dysphoria subscale and Ham-D scores at Week 4 was 0.837 (p<0.01), and the correlation coefficient between the NPI Anxiety subscale and Ham-A scores was 0.763 (p<0.01).
A comparison of the mean scores in the NPI-4W according to the presence or absence of vascular risk factors (HTA, diabetes, hypercholesterolemia, smoking), alcohol consumption, and psychiatric antecedents (depression, anxiety, or psychotic disorders) did not reveal any statistically significant differences. There were also no statistically significant differences on any of the NPI subscales regarding stroke location or size (lacunar versus nonlacunar infarcts).
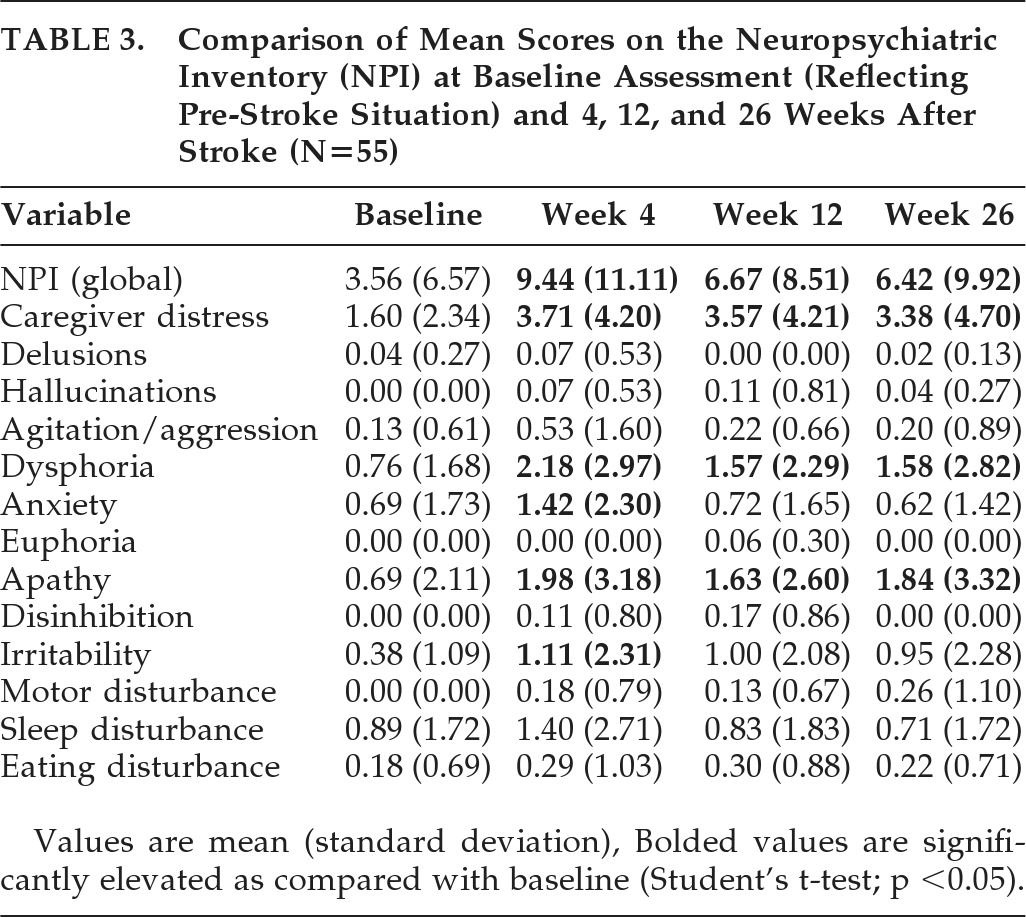
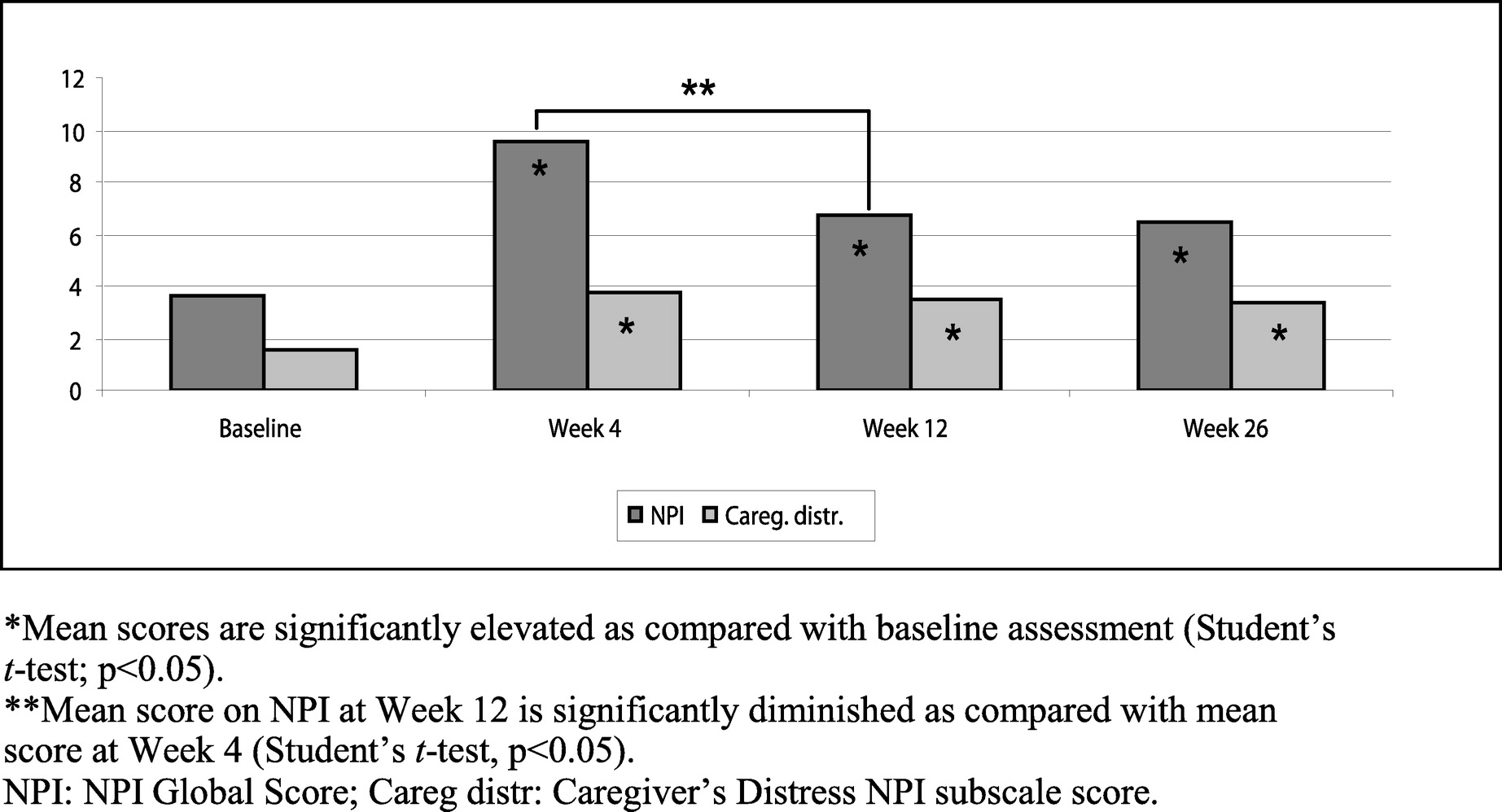
Fifty-five patients completed the study up to Week 26 (
Table 3,
Figure 1). In this population, the mean NPI Global score and the mean scores for the Caregiver's Distress, Dysphoria, and Apathy scales were significantly increased (p<0.05) at Weeks 4, 12, and 26, as compared with the baseline NPI. However, the mean NPI Global score and Anxiety score significantly decreased between Weeks 4 and 12. There were no statistically significant differences in the mean NPI Global score or in any NPI subscale between Weeks 12 and 26.
Correlation With Other Variables
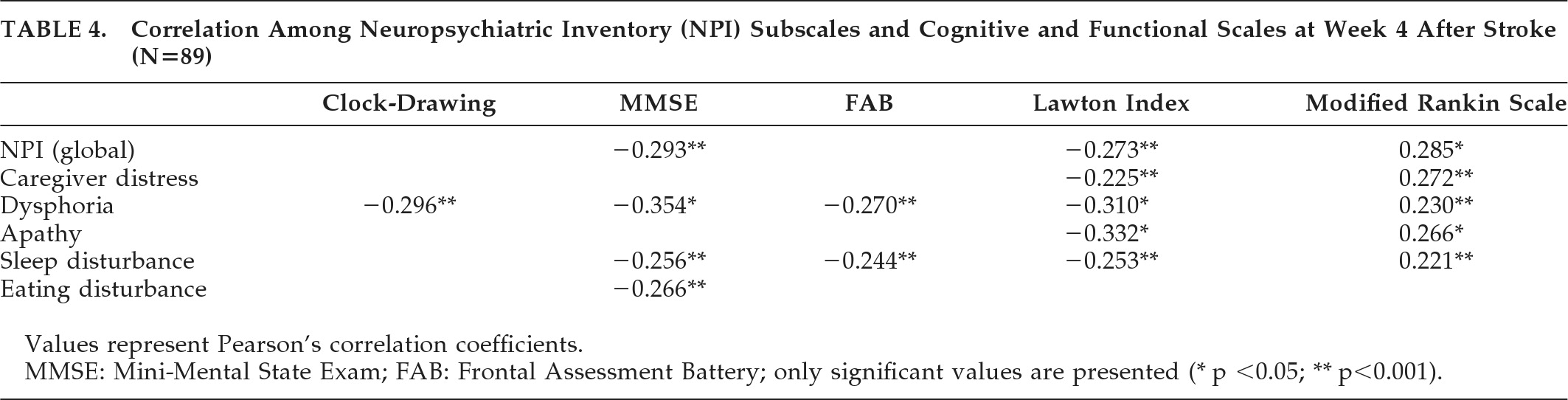
Table 4 shows the NPI-4W subscales that correlated significantly with cognitive and functional variables at Week 4. The Dysphoria score correlated negatively with more cognitive (Clock-Drawing Test, MMSE, and FAB) and functional (Lawton Index and Rankin Scale) scales. On the other hand, functional variables were negatively correlated with more psychopathological variables, including Caregiver's Distress.
In the group of patients who completed the follow-up (N=55), apathy was found to be significantly correlated with the Lawton Index (p<0.001) and Rankin score (p<0.05) at Week 12. At Week 26, the Lawton Index was significantly correlated with Dysphoria (p<0.05) and Apathy (p<0.001) scores, but there were no other correlations between psychopathological variables and the rest of the cognitive and functional scales.
Predictive Factors
Predictive factors for the occurrence of early and long-term PSs after stroke were determined by means of a backward, stepwise linear-regression analysis, which produced explanatory models for some of the psychopathological variables. The NPI Global score and NPI subscales scores at 4 (NPI-4W) and 26 weeks (NPI-26W) after stroke were introduced as dependent variables. The following variables, which could be assessed at the moment of admission, were included as independent variables: baseline NPI Global score and baseline NPI subscales (reflecting previous psychopathological status), psychiatric antecedents (depression, anxiety, or psychotic disorder), demographic characteristics (age, gender, and years of formal education), medical antecedents (vascular risk factors, smoking, and alcohol consumption) and stroke location (right hemispheric, left hemispheric, and brainstem/cerebellum). Qualitative variables were recodified as dummy variables to be introduced in the linear-regression models.
The following NPI-4W subscales produced valid regression models: Agitation, Dysphoria, Apathy, Disinhibition, Irritability, Motor Disturbance, Sleep Disturbance, and Eating Disturbance (
Table 5). Predictive factors for PSs at 4 weeks were younger age, male gender, right-hemispheric location of the CI, and baseline NPI scores. Younger age was a predictive factor for Agitation and Disinhibition, whereas male gender was a predictive factor for Agitation and Irritability. Right-hemispheric location of the CI predicted the development of Dysphoria, Apathy, Disinhibition, and Sleep Disturbance, although its coefficient value was only significant for Sleep Disturbance.
The related baseline NPI subscale score was a predictive factor for the behavior studied at 4 weeks after stroke in every model except in Disinhibition and Sleep Disturbance (e.g., a high baseline NPI score for Dysphoria was a risk factor for having a high score for Dysphoria in the NPI-4W). Apathy at Week 4 was also predicted by a higher score in other baseline NPI subscales (Agitation and Dysphoria). Moreover, a history of hazardous/harmful alcohol consumption was a risk factor for Eating Disturbance, whereas antecedents of depression predicted the occurrence of Sleep Disturbance.
At 26 weeks after stroke, we found valid regression models for Agitation, Dysphoria, Anxiety, Apathy, Irritability, and Sleep Disturbance (
Table 5). Antecedent depression, right-hemispheric location, and scores in the baseline NPI subscales of Agitation, Anxiety, Irritability, and Dysphoria emerged as predictive variables in these models. Right-hemispheric location persisted as a predictive factor for Dysphoria and Apathy, with a significant coefficient value for Dysphoria (p=0.03). Previous history of depression was a risk factor for Dysphoria and Agitation on the NPI-26W, but it made Irritability less probable.
The percentage of variance explained was higher at 26 weeks than at 4 weeks in the models for Agitation, Dysphoria, Apathy, and Irritability, with more than 50% of the variance explained by the models of agitation and dysphoria.
DISCUSSION
Nearly 70% of patients with CI presented some grade of psychopathology in the 4 weeks after stroke onset, as assessed by means of the NPI through a reliable informant. This percentage fell to 55.5% 6 months later. Dysphoria and apathy were the most frequent and persistent symptoms, followed by anxiety, irritability, sleep disturbance, and agitation. These results are consistent with previous reports,
6,26,27 although some differences were present in the frequency of specific symptoms, probably due to methodological factors.
The same percentage of patients had detectable PSs at 4 and 12 weeks after stroke, but the intensity of these symptoms, determined by the mean NPI global score, showed a significant decrease by Week 12 and afterward remained almost unchanged.
In this study, the NPI was used not only for evaluating poststroke affective and behavioral disorders, but also to assess previous psychopathology, in order to evaluate its influence on the occurrence of PSs after stroke. Linear-regression analysis showed that the corresponding subscale score in the baseline NPI was a predictive factor for the most frequent symptoms after stroke, including dysphoria and apathy. Therefore, the development of some specific PSs probably depends, to some extent, on patients' previous affective and behavioral status.
Correlation analysis showed that PSs were related to patients' cognitive and functional status in the early poststroke period. However, in the follow-up, we observed that the only correlations remaining at Week 26 were those between dysphoria and apathy with one of the functional scales (the Lawton Index).
Using linear-regression analysis, we attempted to find out which factors of those that were assessable at stroke onset could be used to predict the occurrence of poststroke PSs. Younger age and male gender were predictive factors for disruptive behaviors (agitation, irritability, and disinhibition) during the first month after stroke. Right-hemispheric location and related baseline NPI subscale scores were the most consistently encountered factors across different models. It must be noted that right-hemisphere location and baseline dysphoria predicted the occurrence of dysphoria both at 4 and 26 weeks after stroke; therefore, these factors can be considered reliable predictors of the development of depressive symptomatology after a CI. Furthermore, right-hemisphere location also predicted the occurrence of apathy both in the early and delayed period after stroke.
In the first month poststroke, right-hemisphere location of CI was also a predictive factor for disinhibition and sleep disturbance. It has been reported that changes in sleep structure, with a reduced REM-to-NREM ratio, are more frequent in patients with right-sided CI;
27 such alterations could contribute to the occurrence of nocturnal behavioral symptoms.
Other reports have also described right-hemisphere location of CI as a risk factor for some PSs, including depression, agitation, and apathy.
28–30 However, in contrast to our findings, most studies have reported an association between left-hemisphere lesions and depression in the early poststroke period, whereas poststroke depression in the chronic phase seems to be more frequent in patients with right-sided lesions.
31 Several reasons have been postulated to explain this discrepancy, including different pathogenetic mechanisms for depression in the acute and chronic phases of stroke, but the measures used to assess depressive symptoms may also have an influence. Patients with right-hemisphere lesions may have indifference to their own symptoms, particularly in the acute phase, a fact that can result in underreporting of depressive symptoms in self-report measures.
31 On the other hand, patients with left-hemisphere lesions frequently present with aphasia, which may be a limitation for the psychopathological evaluation. The NPI, which has been the main tool for PSs assessment in our study, may be adequate for this type of patient, as it obtains information through the caregiver, and the questionnaire refers to both verbal and nonverbal patients' expressions of a depressed mood. In our study, the assessment with the NPI could be completed in patients with aphasia, but we were unable to administer Hamilton's tests (Ham-D and Ham-A, which are patient-referred) to five individuals with a severe language disorder.
It is also important to note that the NPI is intended to evaluate the presence of PSs, but not to diagnose clinical syndromes, as depression. Thus, it cannot be assumed that patients with detected dysphoria in our study actually have poststroke depression. Nevertheless, the Ham-D, which has been previously validated in stroke patients,
16 was used in our patients, in parallel with the NPI, in poststroke assessments, showing a high correlation with the NPI subscale for dysphoria, which indicates that there was good concurrent validity.
Regarding to the practical consequences of our study, it is clear that previous psychopathological status and psychiatric antecedents are important to predict the development of PSs after stroke, but the adequate instruments for psychopathological evaluation must be defined. NPI could be valid for this purpose, but some clinicians could find it time-consuming. However, some of its subscales did not provide any useful information (on hallucinations, delusions, euphoria, motor disturbances, sleep disturbances) in the population studied and could be avoided in these patients. Moreover, a brief questionnaire form of the NPI, the NPI–Q, has been developed as a more appropriate tool for neuropsychiatric assessment in clinical practice.
32In summary, this study has shown a high prevalence of PSs, mainly depressive symptoms and apathy. These disorders were associated with worse cognitive and functional conditions in the post-acute period, but only with functional status in the long term. PSs were more severe in the first month after stroke, but tended to improve with time until reaching a plateau around the third month. Younger age and male gender are risk factors for disruptive behaviors in the early poststroke period, whereas right-hemisphere location of CI and previous related psychopathology were the best predictive indicators for the occurrence of dysphoria/depression and apathy.