Although pharmaceuticals account for a relatively small fraction of total U.S. health care expenditures (10%) (
1), they represent one of the fastest-growing components of such expenditures (
2). In response to this rapid growth, health care payers increasingly apply utilization controls, including preferred drug lists, dispensing limits, mandatory use of generic drugs, step therapy, and prior authorization (
3,
4). A prior-authorization policy requires physicians to obtain preapproval in order for a patient to receive coverage for nonpreferred, and typically more expensive, medications. Approval often requires documents supporting medical necessity for a nonpreferred agent or prior treatment failure with preferred medications (step therapy). Objective data on the intended and unintended consequences of prior-authorization policies for use of medications and health services are limited, particularly in the area of chronic mental illness (
5–
8).
Bipolar disorder, the sixth leading cause of disability worldwide (
9), is a severe and recurrent condition, with manic and depressive episodes. It affects about 2.6% of the U.S. adult population (
10). On the basis of health care claims, patients with bipolar disorder on average spent $22,110 per year for overall treatment in 2002, 53% of which was reimbursed by Medicaid programs (
11). Pharmacotherapy is the foundation of treatment for the acute and long-term management of bipolar illness (
12,
13). Maintenance treatment reduces risks of relapse, hospitalization, and suicide (
14–
16).
In July 2003 the Maine Medicaid program implemented prior-authorization policies affecting new prescriptions for nonpreferred second-generation antipsychotic and anticonvulsant agents, which are effective treatments for bipolar disorder (
12,
13,
17). Nonpreferred second-generation antipsychotics included olanzapine, olanzapine-fluoxetine, and aripiprazole, and nonpreferred anticonvulsants included lamotrigine, topiramate, gabapentin, brand-name carbamazepine, brand-name valproic acid, oxcarbazepine, and levetiracetam.
Prior-authorization policies may reduce the actual or perceived range of prescribing options and thereby limit the flexibility of physicians in adapting drug therapy to individual patient needs if therapeutic problems arise. There is substantial biological heterogeneity within mental disorders, and psychotropic drugs are less therapeutically interchangeable than drugs in other categories (
2). The tolerability of a particular therapy influences patients' adherence (
18). Our recent controlled study found that the Maine prior-authorization policy significantly increased the risk of medication discontinuation among newly treated patients with bipolar disorder (adjusted relative hazard ratio [HR]=2.28; 95% confidence interval [CI]=1.36–4.33) without appreciable cost savings to the state; nor did the policy increase use of preferred agents or rates of switching to other medications (
6). We also observed similar findings among patients with schizophrenia in a study of prior authorization in Maine Medicaid for second-generation antipsychotic medications (
7).
The longitudinal study reported here examined more broadly the impact of the Maine prior-authorization policy for second-generation antipsychotic and anticonvulsant agents among at-risk Medicaid patients with bipolar disorder who were initiating a new episode of drug treatment. We assessed the association of the prior-authorization policy with medication discontinuation and subsequent changes in the use of nondrug health services, including outpatient visits, emergency room visits, and hospitalization. This study differs from our previous studies (
6,
7) in that it provides empirical evidence about the relative effects of prior-authorization policies among patients with severe and less severe bipolar disorder. We anticipated that the policy would result in treatment disruptions among all patients with bipolar illness and that the disruptions could reflect differences in treatment effectiveness, administrative problems (confusion about the policy or the hassle of seeking preauthorization), or other factors (
5–
8). We also anticipated that disruptions in pharmacotherapy after policy implementation would be associated either with increased use of nondrug health services as a result of increased symptoms or with dropout from the health care system and that these outcomes could reflect differences in patient medication adherence and care-seeking behavior because prior authorization adds another barrier to care for a very vulnerable patient population. Previous research has reported an association between medication nonadherence and reduced frequency of physician visits (
19–
22).
Methods
Data sources
We extracted Maine Medicaid enrollment and claims from the Medicaid Statistical Information System of the Centers for Medicare and Medicaid Services for the study period (January 2001–February 2004). We linked these claims data with equivalent Medicare data for the subset of patients with dual Medicaid and Medicare enrollment by using a unique patient identifier (
23). Pharmacy claims contained the patient identifier, National Drug Code, dispensing date, number of units provided (for example, tablets), and days' supply. Health service claims contained the patient identifier, service code, provider code, beginning and end dates of service, primary and secondary diagnoses, service type (for example, emergency room), and medical setting (for example, psychiatric hospital).
We obtained from Maine Medicaid unique identifiers for community mental health centers (CMHCs) to identify service claims made by CMHCs. To calculate patient-level number of CMHC visits, we used claims from the 14 CMHCs with consistent claims reporting (which represented 85% of all CMHC claims during the study period) to create analytic subgroups of CMHC attenders and nonattenders (defined below). We did not count claims from CMHCs that had irregular patterns of claims, which suggested start-up and discontinuation of services or incomplete data during the observation period.
Study cohorts
Newly treated cohorts.
We included patients in Maine Medicaid who were aged 18 years or older in 2001 and who had at least one inpatient or two outpatient diagnoses of bipolar disorder (
ICD-9 diagnostic codes 296.0, 296.1, 296.4–296.8, 301.11, and 301.13) (
6,
24,
25). We identified patients who were newly treated with any bipolar medication (second-generation antipsychotics, anticonvulsants, or lithium). Although not subject to prior authorization, lithium was included because this agent, like anticonvulsants, is an effective mood stabilizer (
17). The newly treated patients included in the study had no use of bipolar medications in the 90 days before the initial drug dispensing (index date) and had spent fewer than 45 days in institutional settings during that period. Patients were required to be continuously enrolled in Medicaid for eight months before and after the index date.
Using these inclusion rules, we identified two distinct cohorts: the policy cohort, or patients who initiated bipolar medication during the period when the policy was in effect (July 2003–February 2004), and the prepolicy cohort, or patients who initiated medication in an equivalent calendar period one year earlier (July 2002–February 2003). For analytic simplicity, only the first treatment episode for each patient was used; thus an individual was in either the prepolicy or the policy cohort. All patients had eight months of baseline observations. To more precisely characterize patients' preexisting trends in health service use, we calculated visit rates during the seven months before the index date, excluding the immediate preceding month because preliminary analysis showed increases in visits around the time of bipolar drug initiation.
Although our original protocol (
6) included New Hampshire as a comparison state, noncomparability of baseline trends in use of outpatient services precluded a state-level comparison. Instead, we used a historical comparison group design: prepolicy and policy cohorts of Maine Medicaid beneficiaries were comparable in their baseline trends in use of medications and outpatient visits.
Subgroups of newly treated patients.
Within the prepolicy and policy cohorts, we created two subgroups of patients according to their baseline CMHC service use. Because CMHCs specialize in care for adults with the most severe and persistent mental illnesses (
26,
27), we hypothesized that regular receipt of CMHC services would be useful as a marker for illness severity. We identified a group of patients who had at least two CMHC visits during the baseline period (CMHC attenders) and a second group with one or no visits (nonattenders).
Outcome measures
Discontinuation of bipolar drug treatments.
We created person-level daily indicators for each bipolar medication; generically equivalent products were treated as the same medication. Our previously validated definition of discontinuation is having a gap of more than 30 days in availability of any bipolar medication (
6,
7). This gap was measured from the date that the supplies from all prior dispensings were finished, assuming perfect adherence, until the date of a subsequent dispensing. Changes in bipolar medication were not classified as discontinuations. The discontinuation date was defined as the first day of such a gap (
5–
7). Shorter time until discontinuation in the policy cohort compared with the prepolicy cohort would suggest an increased rate of disruption in treatment after policy implementation. As a comparison outcome, we also assessed the use of medications to treat conditions other than bipolar disorder.
Health services utilization.
We measured rates of use of both outpatient and inpatient health services, including mental health visits, emergency room visits, non-mental health outpatient visits, and hospital inpatient stays. Mental health visits included outpatient visits that were associated with any diagnosis of bipolar disorder, depression (ICD-9 codes 296.2, 296.3, 298.0, 300.4, 309.1, and 311), or schizophrenia (ICD-9 code 295) or one-day services in psychiatric hospitals. Outpatient emergency room use was defined as visits to emergency departments that were not connected to an inpatient episode, with at least three days of separation from any hospitalization. Non-mental health outpatient visits were those that were not associated with a diagnosis of mental illness.
Statistical analysis
For all analyses, we used a pre-post design with a historical comparison group. We examined individual-level variations in outcomes before and after drug initiation and then compared the policy cohort to the prepolicy cohort to estimate the policy impact. We used the prepolicy cohort to provide information about the counterfactual inference (that is, what might have happened in the absence of policy change). Separate analyses were conducted for CMHC attenders and nonattenders.
To examine the impact of the prior-authorization policy on the hazard of medication discontinuation, we used Kaplan-Meier survival curves and extended Cox regression models (
6,
28). Time until event was the number of days from drug initiation until discontinuation. We used the supremum test to evaluate the appropriateness of the proportional hazards assumption (
5–
7). Given that patients typically receive a month's supply of medicines in the outpatient setting, we could not identify the exact date of discontinuation within a 30-day period after medication was dispensed. To address this limitation, we used extended Cox proportional hazards models, in which patients were stratified according to the timing of discontinuation (≤30 days versus >30 days) (
6).
To examine the association of the prior-authorization policy with changes in visit rates, we used a difference-in-difference analytic approach. We first measured patient-level numbers of mental health visits, emergency room visits, and non-mental health outpatient visits per month in the periods before and after drug initiation for each patient (two observations). For the subset of individuals who had discontinued bipolar medication, we subdivided the postinitiation period into two segments by date of discontinuation: postinitiation while receiving treatment and postinitiation after discontinuation (three observations). For each individual, we then calculated the differences in visit rates across these phases. Finally, we estimated the policy impact by comparing the differences between the prepolicy and policy cohorts.
We performed a series of linear mixed models with numbers of visits per month as the dependent outcomes, all treated as continuous variables, using the PROC MIXED procedure in SAS, version 9.1.3. Mixed models can account for correlations among repeated measurements of the same individual and are unaffected by unequal numbers of assessments among individuals (
29). The models included a constant term, an indicator for policy or prepolicy cohort, indicators for the two postinitiation time periods with the preinitiation period as the reference, and interaction terms between the indicators for cohort and for postinitiation periods. Coefficients of the interaction terms reflect marginal effects of the prior-authorization policy on visit rates across phases. The models provided fixed effects of all independent variables, including interaction terms, and random effects for the intercept.
To examine the association between the prior-authorization policy and the hazard of hospitalization, we used Kaplan-Meier survival curves and Cox proportional hazards regression (
28). We defined time until event as the number of days from drug initiation until subsequent hospitalization. The models included an indicator for policy.
In both the linear mixed and survival models, we controlled for the following patient-level covariates: age group, sex, dual Medicaid and Medicare enrollment status, and two comorbidity measures—number of distinct non-bipolar medications dispensed and number of hospital admissions at baseline (
6,
30). We followed patients until the end of February 2003 for the prepolicy cohort and the end of February 2004 for the policy cohort. We considered p values less than .05 statistically significant in all analyses. This study was approved by the institutional review board of the Harvard Pilgrim Health Care Institute, which waived consent because our study was conducted using deidentified data from a large administrative claims data set.
Results
Baseline characteristics of patient cohorts
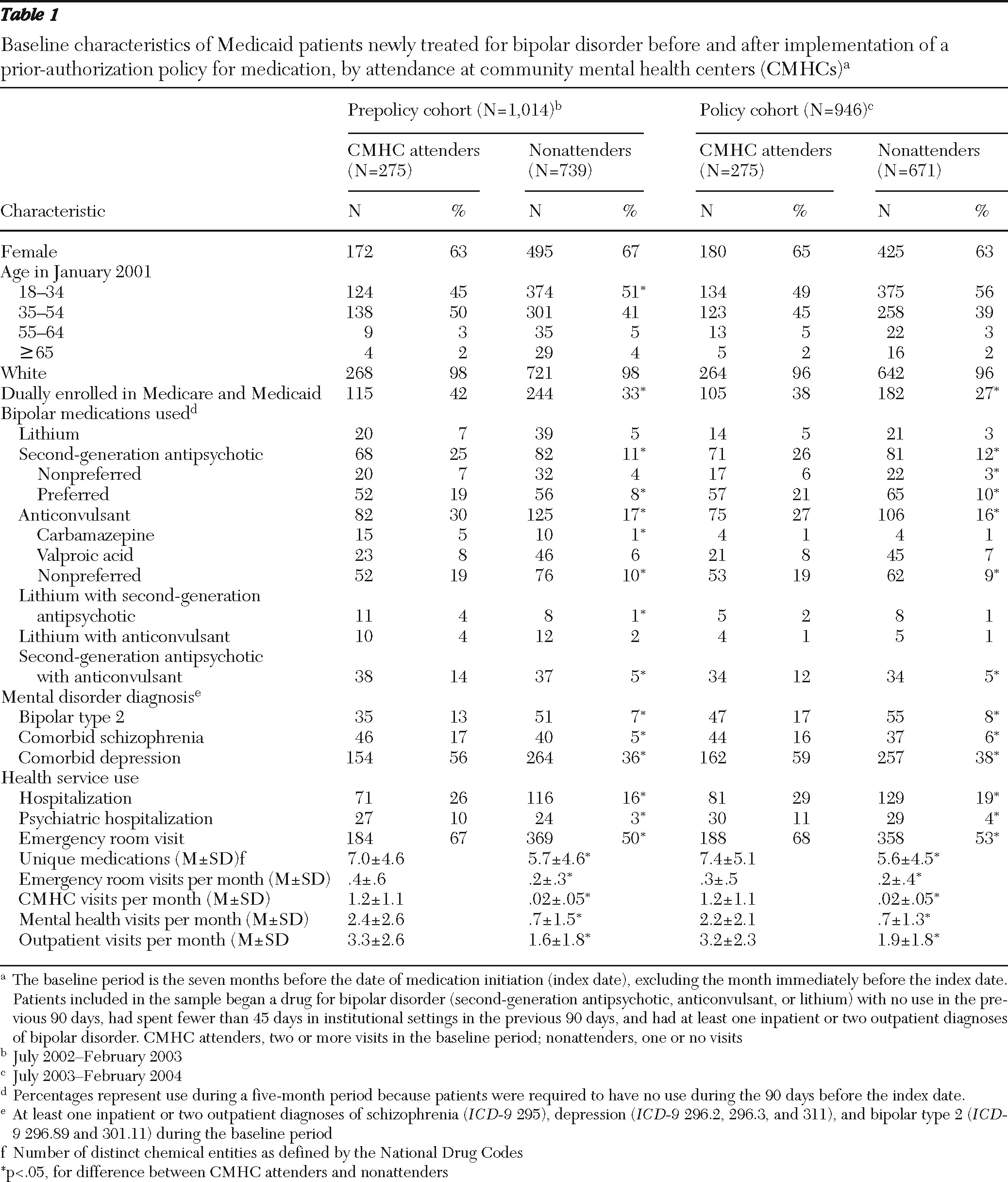
As shown in
Table 1, the total sample included 1,960 newly treated patients, with 1,014 patients in the prepolicy cohort (275 CMHC attenders and 739 nonattenders) and 946 patients in the policy cohort (275 CMHC attenders and 671 nonattenders). Overall, patients who received care at the CMHCs were more likely than nonattenders to have a psychiatric disorder comorbid with bipolar disorder and substantially higher baseline use of psychiatric medications and health services, including psychiatric hospitalizations. Among CMHC attenders, baseline characteristics of prepolicy and policy cohorts were comparable, except for carbamazepine use (5% and 1%, respectively; p<.05). Nonattenders in the prepolicy and policy cohorts were also comparable, except for small differences in dual enrollment in Medicaid and Medicare (33% and 27%, respectively; p<.05).
Discontinuation of bipolar drug treatments
Rates of discontinuation were higher in the policy cohort than in the prepolicy cohort for both CMHC attenders and nonattenders. Among attenders, the discontinuation rate for the policy cohort was 38% (N=104), compared with 31% (N=86) for the prepolicy cohort. Among nonattenders, the respective rates were 41% (N=273) and 33% (N=243).
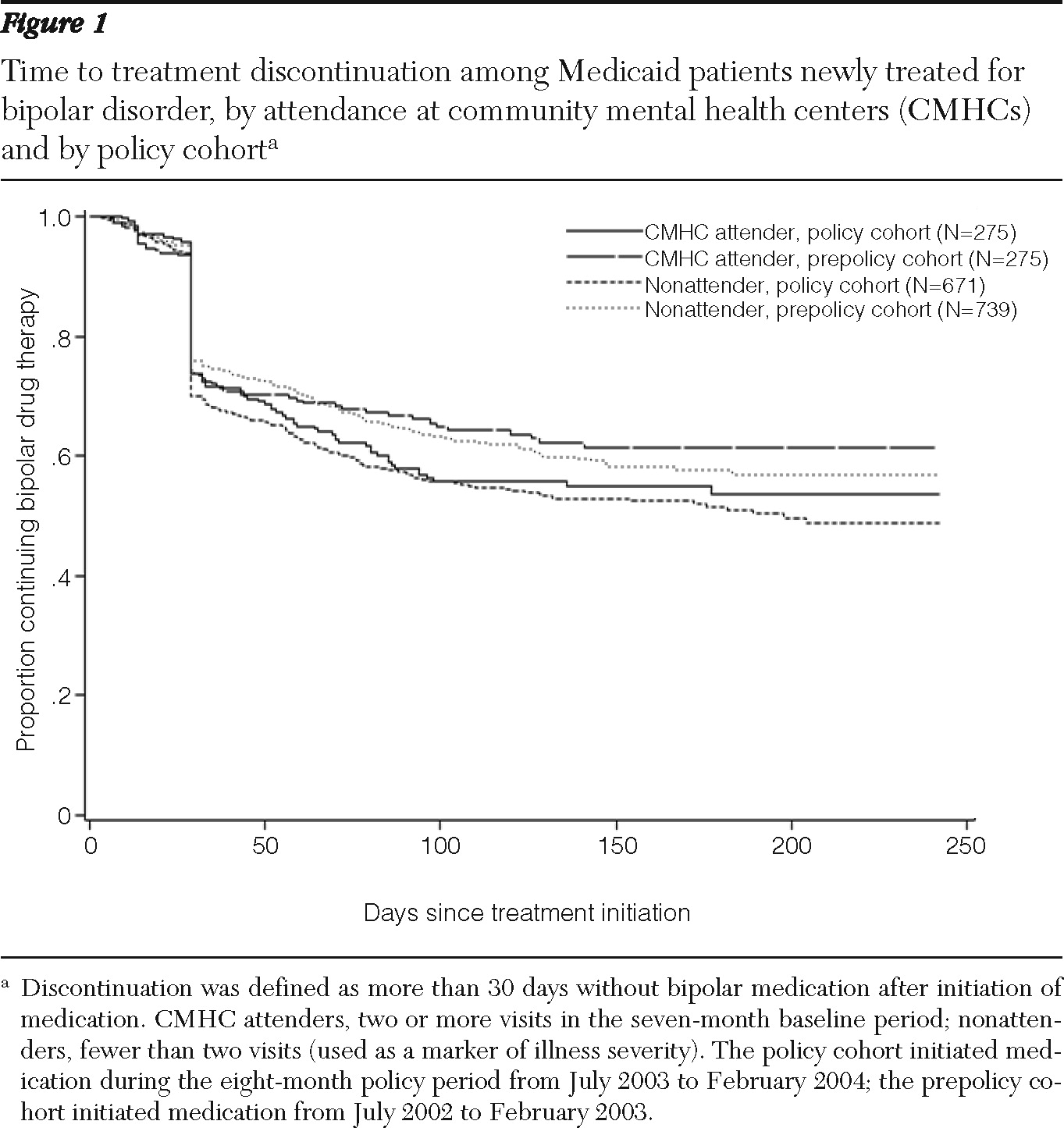
As shown in
Figure 1, the overall risk of medication discontinuation was similar for the CMHC attenders and nonattenders during the eight-month follow-up. Among CMHC attenders, the risk for the policy cohort was similar to that of the prepolicy cohort during the first month of bipolar drug treatment, but a higher risk was found for the remainder of follow-up (adjusted HR=1.73, CI=1.04–2.88; p<.05 (
Figure 1). In particular, this risk was significantly higher among patients who initiated treatment with second-generation antipsychotic medications (HR=2.52, CI=1.20–5.28; p<.05).
Among nonattenders, the policy cohort was more likely than the prepolicy cohort to discontinue medications within 30 days of initiation (HR=1.30, CI=1.05–1.61; p<.05), and this difference persisted until the end of follow-up. Among those who initiated treatment with second-generation antipsychotic medications, the policy cohort had a higher risk of discontinuation than the prepolicy cohort (HR=1.65, CI=1.16–2.35; p<.05). The risk of discontinuation among those who initiated treatment with anticonvulsants did not change significantly for either the CMHC attenders or nonattenders.
During the study period we did not observe any changes in the dispensing rates of drugs used to treat conditions other than bipolar disorder.
Health services utilization
During the postinitiation periods, the mean±SD number of days of observation before discontinuation of bipolar medication was 86±70 for CMHC attenders and 84±68 for nonattenders, and after discontinuation, the mean number of days of observation was 104±54 and 100±60, respectively, until the end of the study.
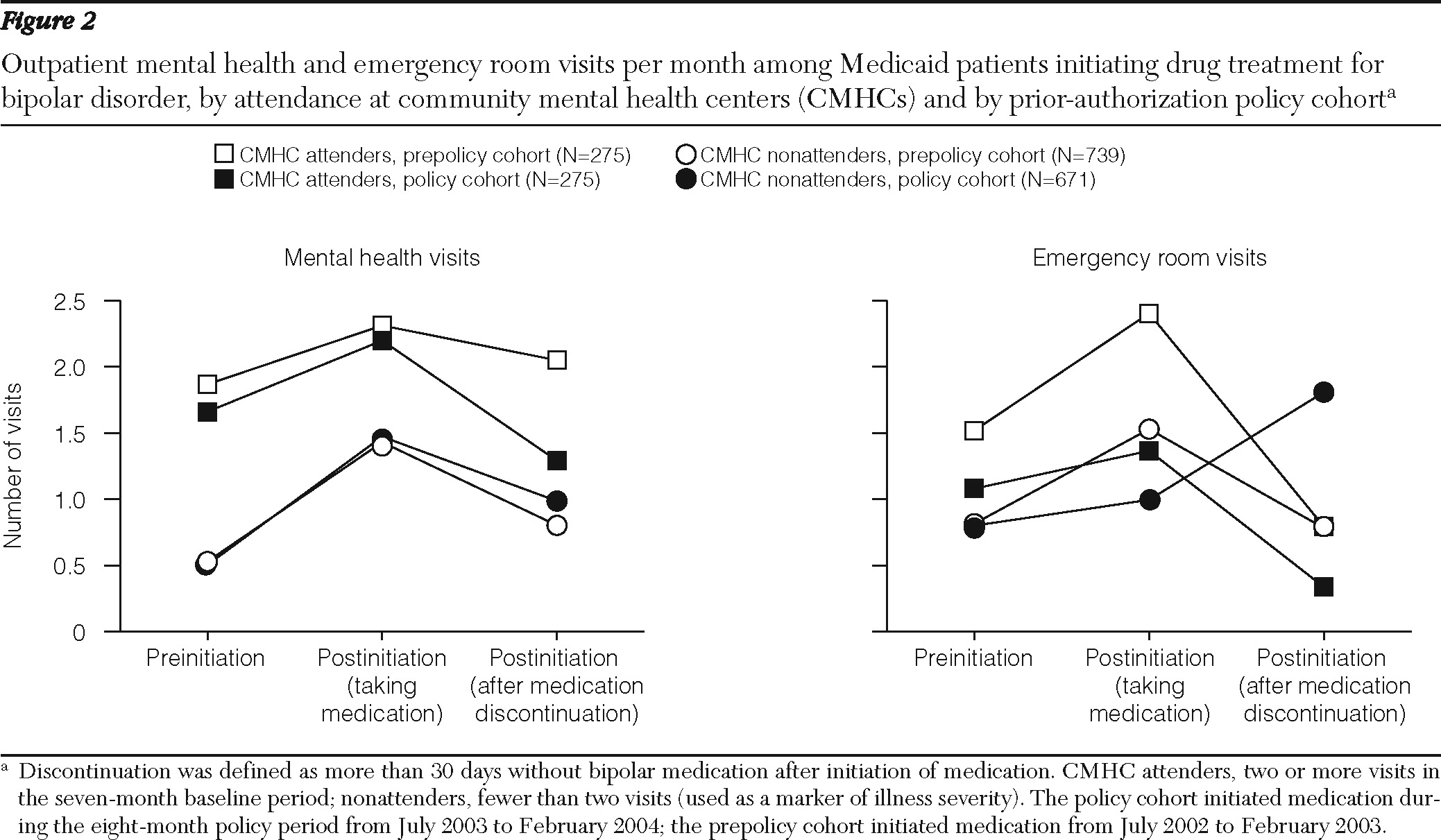
Figure 2 shows the changes in visit rates before and after drug initiation. [A table presenting data on the estimated changes in monthly visits across periods for the prepolicy and policy cohorts is provided in an online appendix to this article at
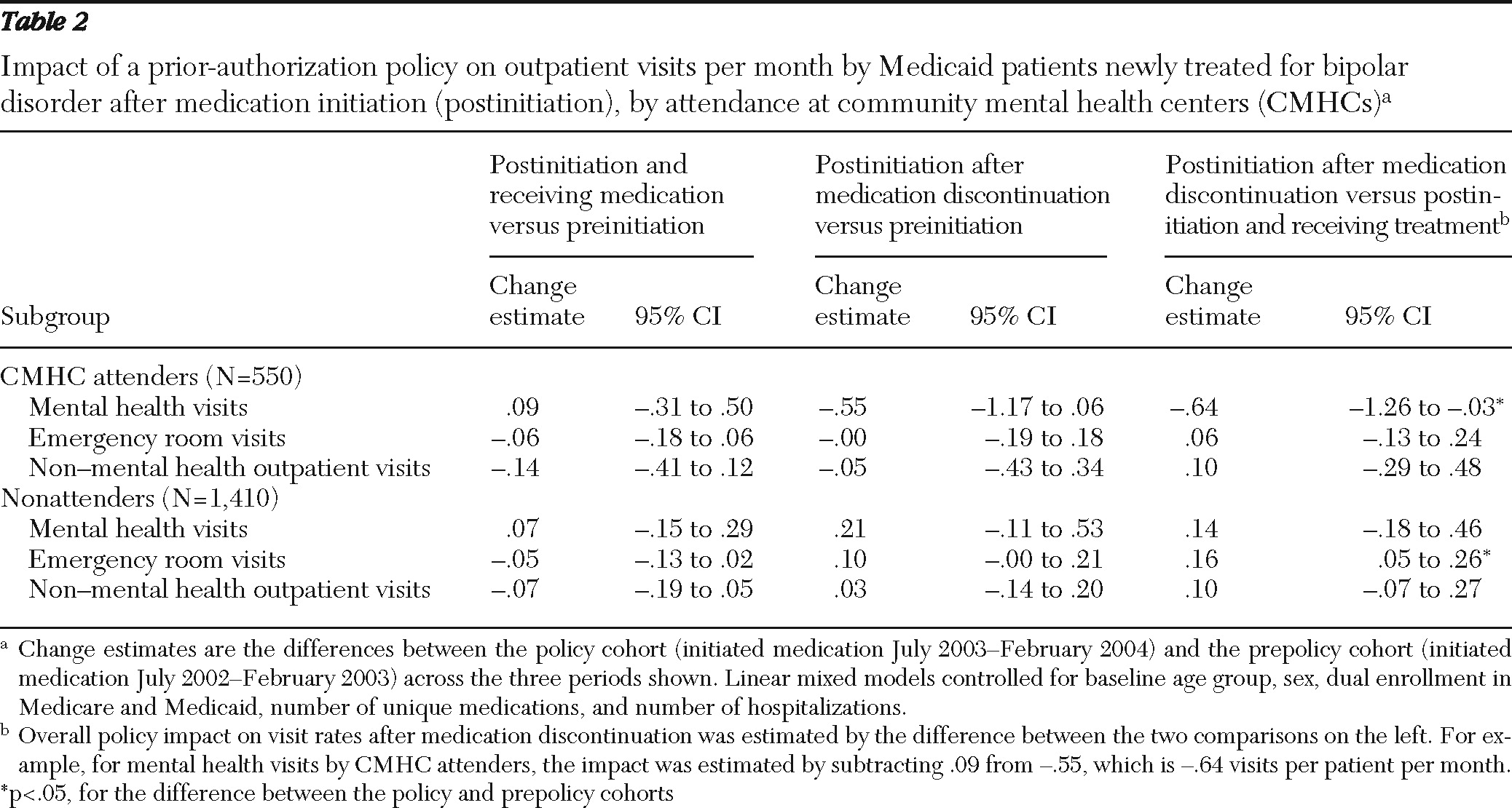
ps.psychiatryonline.org.] Among CMHC attenders, an increase in the rate of mental health visits per month was associated with initiation of medication for both cohorts (prepolicy cohort: .54 visits per patient per month, CI=.25–.82; policy cohort: .44 visits per patient per month, CI=.16–.73) [see online appendix]. Compared with the prepolicy cohort, the policy cohort experienced a large and statistically significant reduction in outpatient mental health visits after medication discontinuation (−.64 visits per patient per month, CI=−1.26 to −.03; p<.05) (
Table 2 and
Figure 2).
Among nonattenders, an increase in outpatient mental health visits for both the prepolicy and policy cohorts was associated with drug initiation followed by reductions in visits after discontinuation, but the differences between the cohorts were not significant (
Table 2 and
Figure 2). However, compared with the prepolicy cohort, nonattenders in the policy cohort experienced a significant increase in emergency room visits after medication discontinuation (.16 per patient per month, CI=.05–.26; p<.05) (
Table 2 and
Figure 2).
As shown in
Table 2, no differences were found between the prepolicy and policy cohorts in rates of change in non-mental health outpatient visits for either CMHC attenders or nonattenders.
Results of the Cox proportional hazards model suggest that during the eight-month follow-up, the policy had no detectable impact on the risk of hospitalization among CMHC attenders (HR=1.14, CI=.78–1.65) and nonattenders (HR=1.26, CI=.93–1.69).
Discussion
Our previous study found that the Maine prior-authorization policy for second-generation antipsychotic and anticonvulsant agents was associated with increases in medication discontinuation (
6). The study reported here suggests that the prior-authorization policy had a differential impact on medication discontinuation and use of health services among newly treated patients with bipolar disorder. Among patients who had at least two CMHC visits during the baseline period (CMHC attenders), the policy was associated with both an increase in rates of disruption in pharmacotherapy and a greater decline in outpatient mental health visits after drug discontinuation. The policy impact was immediate among CMHC nonattenders (those with one or no visits), who had a higher risk of medication disruption within the first month of treatment and relative increases in emergency room visits after medication discontinuation. CMHC attenders appeared to remain adherent to bipolar medications longer than nonattenders, which may reflect monitoring of patients with more severe illness in the CMHC setting, although rates of discontinuation were ultimately higher in both groups at the end of follow-up.
Prior-authorization policies are controversial because they can introduce barriers to the timeliness or appropriateness of treatment initiation or selection (
5–
8,
31). It is a concern that the policy was associated with increased medication discontinuation among all patients with bipolar disorder. Medication nonadherence is already a frequent problem among patients with bipolar disorder and can be as high as 65% (
32), which is higher than that among patients with schizophrenia (40%–50%) (
32) and patients with nonpsychiatric conditions (an average of 25%) (
33). Because of the longevity of bipolar illness, management of such patients requires routine care and maintenance treatment to minimize residual symptoms and reduce risk of recurrence (
34–
36).
We found a greater reduction in rates of outpatient mental health visits after medication discontinuation among CMHC attenders in the policy cohort. Because prior authorization is administratively cumbersome for both patients and doctors, it may create unintended barriers that influence patient compliance. Fewer outpatient mental health visits after medication discontinuation could represent a significant concern if it compromises continuity of care of these seriously ill patients. Further, our results suggest that CMHC nonattenders in the policy cohort used emergency room services more frequently after medication discontinuation. This may indicate an increased incidence of symptoms among individuals not receiving treatment. An alternative, potentially more plausible, explanation is that this reflects attempts by patients to manage medication access issues by visiting outpatient emergency rooms, which are a possible setting of care for patients who do not regularly attend CMHCs. Although the Maine prior-authorization policy reduced pharmacy reimbursement (by about $27 per patient over the eight-month policy period [6]), it is unlikely that these small savings offset the cost of increases in acute care services, which impose greater societal treatment costs for this group of patients.
We did not detect any effect of the policy on rates of hospitalization. However, interpretation of this outcome is limited by the lack of data on state psychiatric hospitalizations, the small sample, and the short (eight-month) follow-up, which was the result of the sudden discontinuation of Maine's prior-authorization policy in March 2004 after reports of adverse effects associated with the policy (
7,
37). Longer-term consequences of prior-authorization policies for health and economic outcomes could not be reliably ascertained from this study.
Several limitations merit discussion. First, continuously enrolled patients can represent a selected subgroup of the Medicaid population; however, such a sample was necessary to obtain complete information about outpatient medication use. A sensitivity analysis in which we included individuals continuously enrolled for at least 90% of the study period had an additional 173 participants: 50 CMHC attenders (prepolicy cohort, N=21; policy cohort, N=29) and 123 nonattenders (N=53 and N=70, respectively). This analysis found no changes in the characteristics of study groups or in the results. Second, because we did not have access to medical records and state psychiatric hospital data, we used regular receipt of CMHC services as a marker for disease severity; however, some individuals may be misclassified. Notwithstanding this limitation, there is a need to examine the impact of cost-containment policies by patients' illness severity.
Third, 77 individuals who had visited the excluded CMHCs at baseline were categorized as nonattenders by our study definition (prepolicy cohort, N=30; policy cohort, N=47). To address possible misclassification, we conducted a sensitivity analysis in which we excluded these individuals. We observed no changes in the characteristics of the nonattender group or in our results. Fourth, we could not observe changes in visits to state psychiatric facilities that do not bill Medicaid. Finally, changes in visit rates might have been attributable in part to patients' care-seeking behavior and the care management patterns of CMHCs; however, the use of the prepolicy cohort as a counterfactual comparison in our analyses of policy effects is likely to have minimized such confounding.
Conclusions
Introduction of the Maine prior-authorization policy for second-generation antipsychotic and anticonvulsant agents was associated with increased rates of medication discontinuation among vulnerable groups of patients who were initiating new episodes of treatment for bipolar disorder. After these discontinuations, reductions in outpatient mental health visits were observed among the sickest patients treated in CMHCs, along with increases in emergency room visits among other patients. These small but meaningful unintended policy effects raise concerns about quality of care. Long-term consequences of prior-authorization policies on patient outcomes warrant further investigation.
Acknowledgments and disclosures
This study was funded by grant 63213 from the Robert Wood Johnson Foundation's Changes in Health Care Financing and Organization Program (Dr. Soumerai, principal investigator) and was conducted at the Department of Population Medicine, Harvard Medical School, and the Harvard Pilgrim Health Care Institute. Dr. Lu acknowledges funding from a fellowship in pharmaceutical policy at Harvard Medical School and a Sir Keith Murdoch Fellowship from the American Australian Association. Dr. Adams' participation was supported by the Kaiser Permanente Northern California Community Benefit program. Dr. Ross-Degnan, Dr. F. Zhang, and Dr. Soumerai are investigators in the HMO Research Network Center for Education and Research in Therapeutics, which is supported by the U.S. Agency for Healthcare Research and Quality.
The authors report no competing interests.