Increasingly sophisticated models of symptoms in schizophrenia have been proposed (
1). The relationship of cognitive impairment to clinical symptoms has been widely debated, with some recent factorial studies suggesting it to be an independent dimension (
2). Although no clear pattern linking specific cognitive deficits with symptoms has been consistently established, many studies have examined the correlation between performance on certain neuropsychological tests in schizophrenic patients and their positive or negative symptoms.
Most often, measures from standard neuropsychological batteries have been found to be correlated with negative symptoms (
3–
5). Evidence from brain metabolism studies has linked negative symptoms to hypofrontality (
6,
7). Since frontal lobe involvement has been linked to deficient executive control functioning in schizophrenic patients (
8), negative symptoms in these patients would be expected to be particularly associated with tasks relying heavily on executive components, e.g., effortful operations.
On the contrary, positive symptoms have only rarely been found to be correlated with standard neuropsychological measures. The few relevant studies suggest that positive symptoms may, rather, be linked to a function not usually tested by standard batteries but assumed to be specifically impaired in schizophrenia: reality monitoring, i.e., the ability to discriminate between internally and externally generated events (
9). An association between a deficit in reality monitoring and positive symptoms has been found in multiple studies (
10–
16).
One framework for integrating different roles of positive and negative symptoms is Frith's pathophysiological model of schizophrenia (
17). Frith suggests that negative symptoms stem from a failure to respond, whereas positive symptoms are linked to a failure to monitor an internally produced response. Accordingly, some studies have revealed different patterns of association with neuropsychological measures for positive compared to negative symptoms (
18–
20).
A key function that has repeatedly been found to be impaired in schizophrenia is memory (
21). It has been linked to various symptom clusters, including negative symptoms (
22), anergia (
23), positive symptoms (
24), and formal thought disorder (
22) or has been found to be unrelated to any symptoms (
4,
25) or to be characterized by a dissociated pattern of relations with symptoms (
26).
Although the focus of the previously mentioned studies has generally been positive, negative, or disorganized symptoms, recent models have also emphasized the role of depressive symptoms in schizophrenia (
2,
27). It has widely been reported that depressed patients have memory impairment (
28), and it has been demonstrated that they are deficient in taking advantage of organization at the encoding stage (
29). The degree of the memory deficit has been shown to increase with severity of depressive symptoms (
30). Depressive symptoms have similarly been found to affect memory performance in various other populations (
31–
34). Among the different components of memory, it has been suggested that it is the effortful processes that are impaired in depression (
29,
32,
35). Yet, as far as we know, the role of severity of depressive symptoms on memory has not been systematically investigated in schizophrenia.
The purpose of this study was therefore to elucidate the correlates of memory impairment in schizophrenia with positive, negative, and depressive symptoms. However, memory is a complex system relying on various components, any of which may be differentially affected by symptoms. Therefore, we attempted to break down overall memory performance into some of its component functions (free recall in long-term memory, recognition, implicit memory, short-term memory) and stages (encoding, storage).
We subdivided the encoding stage into superficial encoding and deep encoding. This distinction is relevant since the efficiency of memory is dependent on the depth of encoding (
36). Superficial encoding (learning items by rehearsal in either short-term or long-term memory) was assumed to rely more on automatic than effortful processes (
37). On the other hand, deep encoding (processing of the semantic organization) was assumed to be an essentially effortful process. Free recall and recognition performances have been found to be correlated with the degree of categorization at encoding in schizophrenia (
25,
38). Therefore, any symptoms linked to the indices of deep encoding should also show links with free recall and recognition performances.
Finally, the storage stage of memory was assessed by comparing immediate and delayed recall. As far as we know, however, no study has investigated any symptomatic correlation with the storage stage, so we made no specific hypotheses about it.
Since we wanted to study symptomatic correlates of memory within the framework of the previously mentioned dichotomy between the failure to respond and the production of erroneous responses, we distinguished between these two types of memory dysfunction in our results. Failure to respond was indexed by poor efficiency in the different memory tasks. Erroneous responses were defined as intrusions (recollections of words not previously presented) and perseverations (recollections of words presented in previous lists) in the free recall task and as false alarms (recognitions of words not previously presented) in the recognition task. All three can be thought of as stemming from a reality monitoring defect, i.e., an error regarding the source of the information (
17). Intrusions and false alarms reflect a belief in the reality of an event that has not occurred. Perseverations reflect the failure to notice that the response has already been emitted or the failure to place the event in its appropriate context, i.e., in its list.
Accordingly, our hypotheses were as follows: 1) depressive symptoms and negative symptoms would be linked to measures reflecting effortful processing: indices of deep encoding as well as free recall and recognition scores, and 2) positive symptoms would be linked to erroneous memory responses.
METHOD
Participants
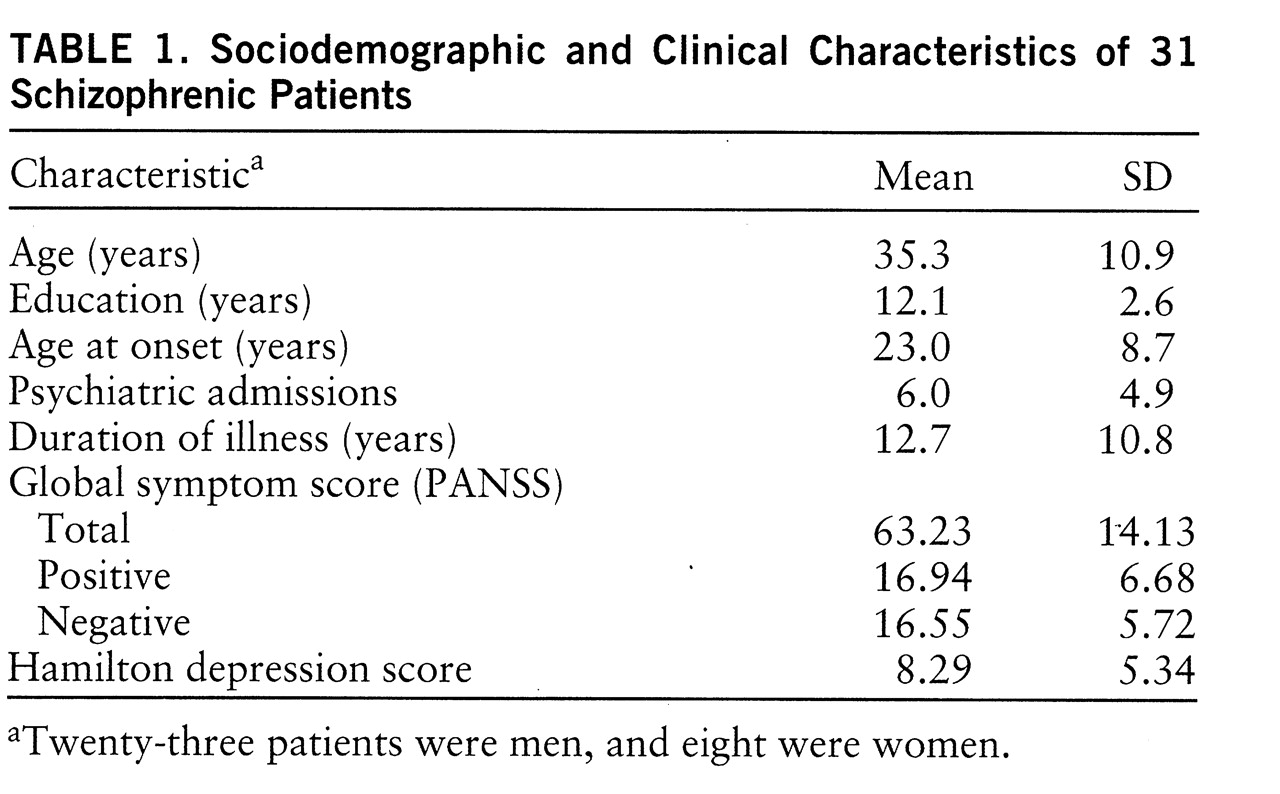
Forty inpatients who met DSM-IV criteria for schizophrenia underwent the memory task. They consisted of 27 men and 13 women with a mean age of 33.7 years (SD=10.5) and a mean educational level of 12.5 years (SD=2.7). They were recruited from the Schizophrenia Research Unit at the New York State Psychiatric Institute and Creedmore Psychiatric Center. All were on regimens of either typical (haloperidol, fluphenazine) or atypical (clozapine, risperidone) neuroleptics and were receiving fixed doses at testing. Written informed consent was obtained for each patient. However, clinical data were available for only 31 of them (
table 1 provides sociodemographic and clinical information). The analyses reported later in this article were carried out on the smaller subset unless otherwise noted.
Clinical Rating
Scores for positive and negative symptoms were taken from the Positive and Negative Syndrome Scale (PANSS) (
39). Severity of depression was assessed by the Hamilton Depression Rating Scale (
40). One of three clinicians completed the diagnostic (Diagnostic Interview for Genetic Studies) (
41) and symptom evaluations. Raters were trained to high reliability with each other (i.e., kappa greater than 0.80 for individual symptom ratings and 95% agreement on diagnosis). The experimenter (G.B.) was blind to all ratings of symptoms throughout patient testing. Similarly, the raters were blind to the memory test scores.
Materials
Six equivalent lists of 16 concrete, highly frequent (
42) monosyllabic or bisyllabic words were constructed. Two of the lists were organizable in four different semantic categories, with four words in each. The 16 words of these organizable lists were randomized. One of the nonorganizable lists and one of the organizable lists were used for immediate free recall. One of each was also used for delayed free recall. The two remaining nonorganizable lists were used for the recognition task. Two recognition sheets were prepared, each including the 16 words of the recognition list mixed with 16 distractors equivalent in length and frequency. Finally, a stem completion sheet was prepared to assess implicit memory. It displayed the first three letters of 20 of the words presented in the two nonorganizable lists used for free recall.
Procedure
Free recall. Subjects were given a sheet displaying a list of 16 words and were told that they had 30 seconds to learn it. They were required to read the words aloud at least once, so that the experimenter could make sure that all the words had been processed. Then, either immediately or after a 1-minute delay, they were given a sheet with 16 empty spaces and were required to write down as many words as they could remember from the list, in any order and without any time limit. In the delayed condition, subjects were asked to read out loud a passage of a book for 1 minute. This procedure was repeated for the four lists. All conditions were counterbalanced. Subjects were not warned of the possible organization in semantic categories during the learning stage.
The total number of recalled words in the four lists was used as a measure of memory efficiency in free recall. In addition, superficial encoding was assessed in each of the four lists by a sequence index (
38), defined as the proportion of items recalled in their order of presentation out of the total number of recalled items. This index reflected the propensity to learn the words of the list by heart, without using their possible semantic organization. The number of items per sequence was computed for each list, reflecting the efficiency of this superficial encoding strategy. A single mean sequence index and a single mean number of items per sequence across the four lists were computed. Deep encoding was assessed by a categorization index, computed on the recall of the two semantically organizable lists. It was defined as the number of items following an item of the same semantic category in the recalled list, divided by the number of such possible associations. It reflected the propensity to encode words according to their semantic properties, which indicates deep encoding (
43). The efficiency of this strategy was then assessed by the total number of categories recalled and the total number of recalled items within the category. These indices were averaged across the two organizable lists.
The number of perseverations (recall of a word from a former list) and intrusions (erroneous recall of a word that had not been presented) was also recorded. Both of them were referred to as erroneous memory responses.
Implicit recall. After the recall of the four lists, subjects were given the stem completion sheet. They were instructed to complete as quickly as possible each string of three letters by the first English word that came to mind. They were not told that each stem could be completed by a word that had been previously learned. The number of stems completed by a word that was actually in one of the previously learned lists was recorded.
Recognition. The last two nonorganizable lists were learned in the same conditions as in free recall. After learning, subjects were given the recognition sheet and were required to check the words that they recognized from the previously learned list. In order to get a measure of accuracy in recognition independent of response bias, the index of accuracy Pr derived from the Two-High Threshold theory (
44) was computed for both immediate and delayed recognitions. The mean Pr was computed as the measure of efficiency of recognition. The propensity to make false alarms rather than omissions was assessed by the response bias Br. A higher value of Br corresponded to a greater propensity toward false alarms. Again, these indices were averaged across both recognition lists, and mean Br was used as the index of erroneous memory responses in recognition.
Short-term memory. The forward and backward digit spans of the WAIS (
45) were administered to assess short-term memory. Increasingly long series of digits had to be repeated, either in the same or in reverse order.
Data Analysis
Data analysis was carried out by means of the SPSS for Windows program (
46). The Pearson product-moment coefficient was used for correlations, except for one measure (intrusions) for which the nonparametric Spearman coefficient was used because of nonnormal data distribution. The full results of this memory battery have been reported and discussed in an earlier study (
38) and will not be reported here.
RESULTS
Memory Efficiency
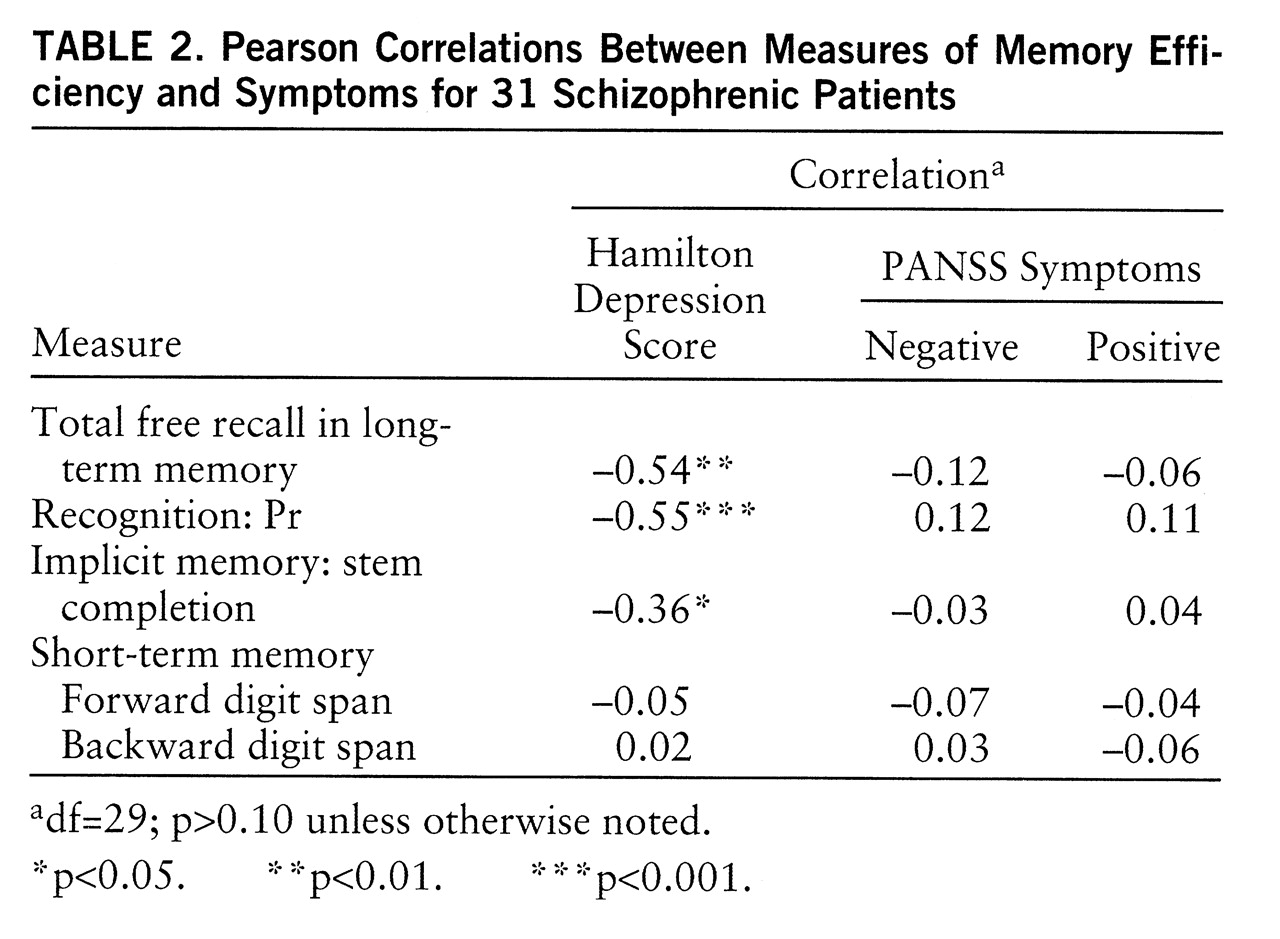
As predicted, the measures of efficiency in free recall and recognition were negatively correlated with severity of depressive symptoms (
table 2). This association also turned out to be true for implicit memory score. Because of the possible overlap between depressive symptoms and positive and negative symptoms, partial correlations were computed. These three correlations of memory efficiency with severity of depressive symptoms remained significant when the score for positive symptoms was partialled out (total free recall: r=–0.55, df=28, p<0.005; Pr: r=–0.61, df=28, p<0.0001; stem completion: r=–0.38, df=28, p<0.05) and when the score for negative symptoms was partialled out (total free recall: r=–0.54, df=28, p<0.005; Pr: r=–0.56, df=28, p<0.001; stem completion: r=–0.35, df=28, p<0.05). However, contrary to what was expected, the memory measures that were assumed to rely on deep processing in free recall and recognition were not significantly correlated with negative symptoms.
Erroneous Memory Responses
In order to test whether erroneous memory responses constituted a factor separate from memory efficiency, we carried out in the larger group (N=40) a principal components analysis with rotated factors (Varimax). Two measures of efficiency of memory—number of recalled words and Pr in recognition—along with the three types of erroneous responses (intrusions, perseverations, and bias toward false alarms), were entered. Two factors were extracted (eigenvalues over 1). One, accounting for 30% of the variance, loaded for number of recalled words (0.85) and recognition discrimination (0.87) but not for intrusions (–0.19), perseverations (0.04), or bias toward false alarms (0.13). The second one, accounting for 33% of the variance, loaded for intrusions (0.74), perseverations (0.80), and bias toward false alarms (0.60) but not for number of recalled words (0.22) or recognition discrimination (–0.19).
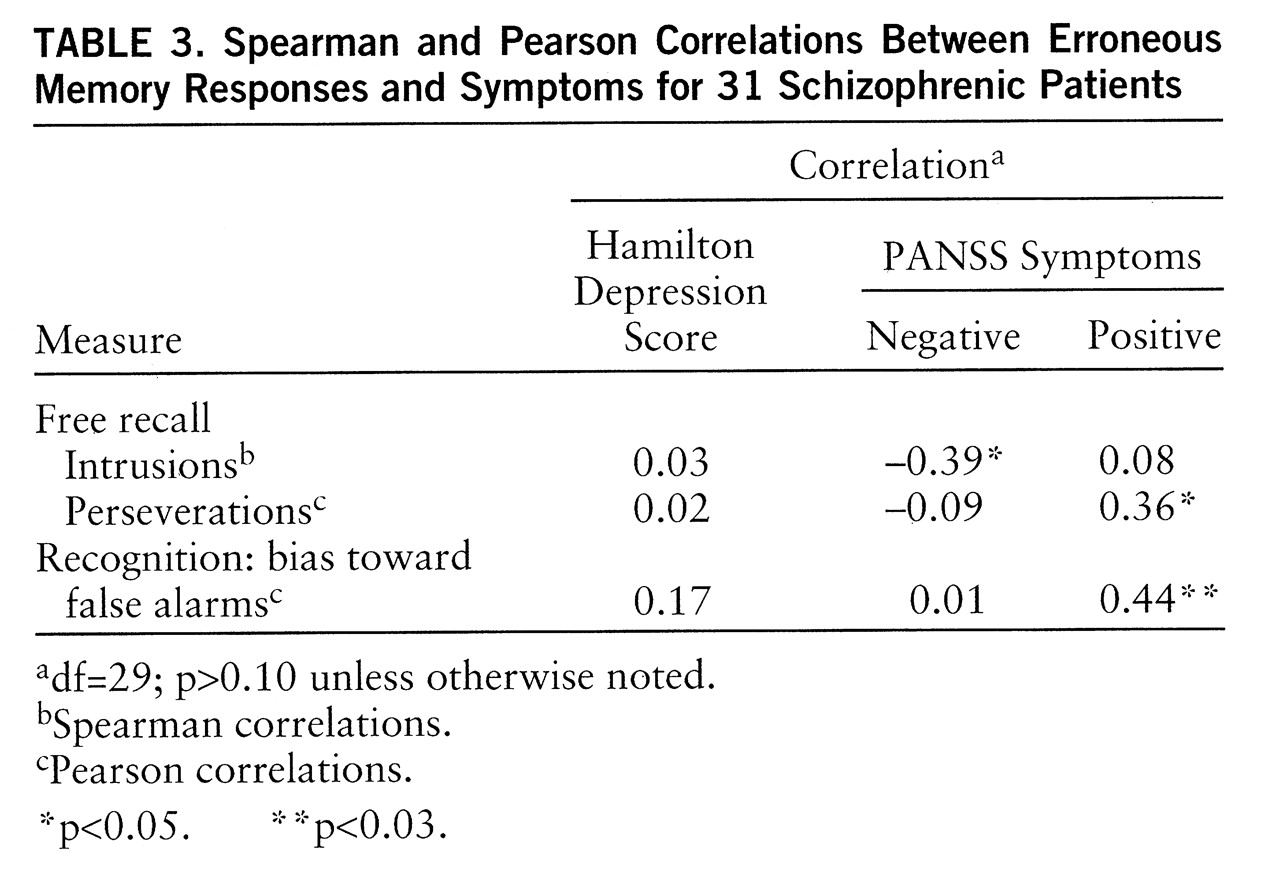
Correlations between erroneous memory responses and clinical measures were computed in the smaller group (
table 3). As expected, perseverations and bias toward false alarms were correlated with positive symptoms. Surprisingly, intrusions were negatively correlated with negative symptoms, indicating that patients who had more negative symptoms made fewer intrusions.
Memory Stages
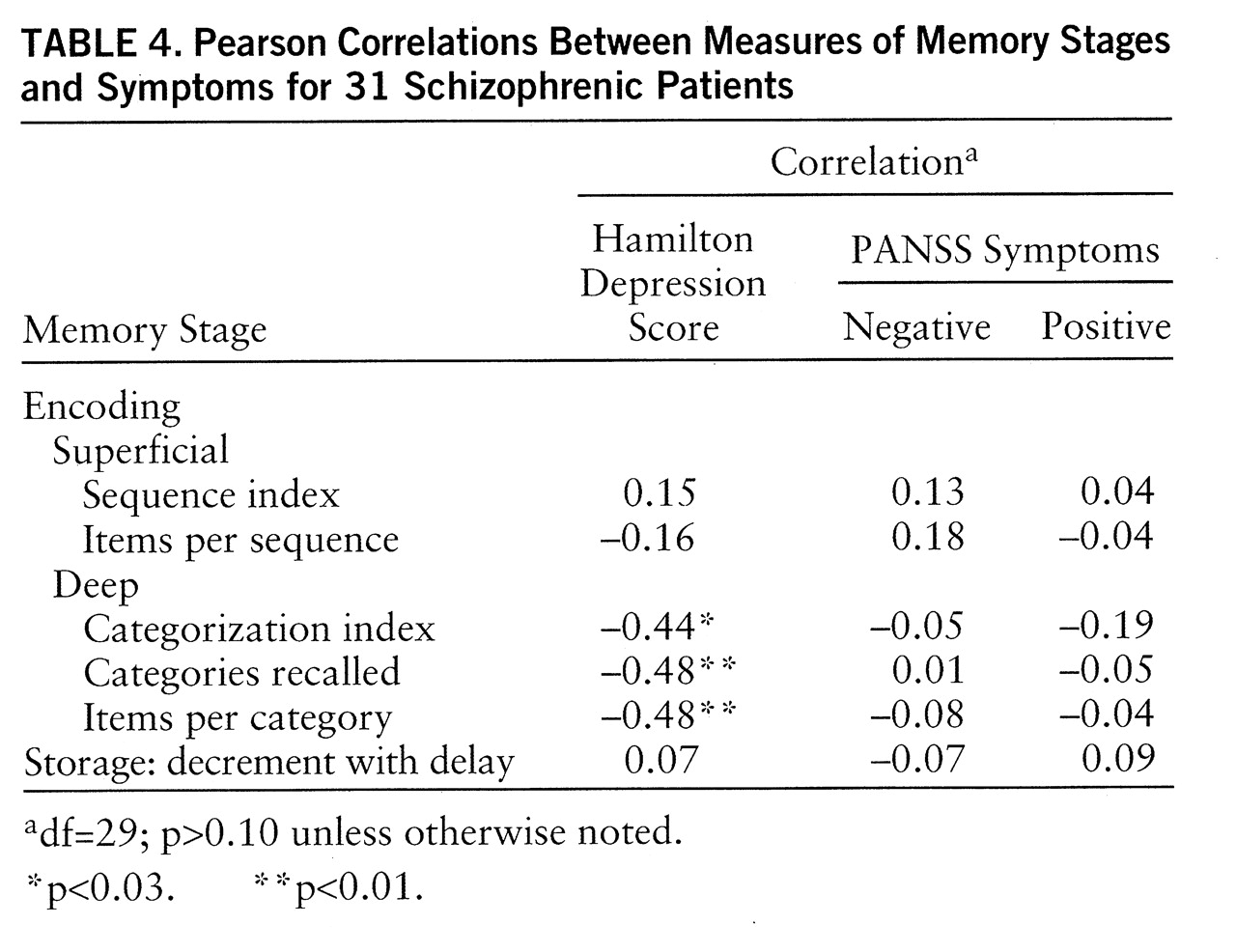
Encoding. As expected, the two indices of superficial processing were not significantly correlated with depressive or negative symptoms. They were not correlated with positive symptoms, either (
table 4). As expected, the three indices reflecting deep encoding were significantly correlated with depressive symptoms. These three correlations remained significant when positive symptoms were partialled out (categorization index: r=–0.41, df=28, p<0.025; categories recalled: r=–0.48, df=28, p<0.01; items per category: r=–0.48, df=28, p<0.01) and when negative symptoms were partialled out (categorization index: r=–0.44, df=28, p<0.025; categories recalled: r=–0.48, df=28, p<0.01; items per category: r=–0.47, df=28, p<0.01). However, contrary to what was expected, these three indices were not correlated at all with negative symptoms.
Storage. The storage stage was assessed by the decrement with delay in free recall. It was not associated with any clinical measure.
DISCUSSION AND CONCLUSIONS
As expected, depressive symptoms were consistently linked with all the measures reflecting deep encoding, even when positive or negative symptoms were partialled out. Thus, the links between severity of depressive symptoms and effortful memory processes previously observed in depressed patients, as well as in other populations, seem to be applicable to patients with schizophrenia.
Our results give a further validation of the most recent models that emphasize the role of depressive symptoms as a distinct dimension in schizophrenia (
2,
27). Depressive symptoms may thus constitute a relatively autonomous dimension linked to memory and possibly other effortful cognitive functions in various populations, whatever the diagnosis. Such a finding is potentially important for the management of schizophrenia. Indeed, it has not been clearly established that currently used neuroleptics routinely improve memory function in schizophrenia; it may be that antidepressants would be a more appropriate pharmacological strategy for this purpose.
In addition, our results suggest that it is relevant for the understanding of memory performance in schizophrenia to differentially consider two types of impairment: the failure to respond and the production of erroneous responses. In this experiment, negative symptoms were not at all associated with the efficiency of any type of memory assessed. This was not in agreement with Frith's suggestion that the failure to respond is linked to negative symptoms, and it seems to contradict a large body of data showing that negative symptoms affect cognitive efficiency. It could mean that the commonly found relationship with negative symptoms in previous studies has been plagued by the existence of subthreshold depressive symptoms, because of behavioral similarity between these dimensions (
47). Alternatively, it could be that the association between negative symptoms and cognitive function does exist but concerns cognitive systems other than memory. Also, our group may be atypical or, for some reason, different from those involved in other studies. Finally, the lack of correlation might stem from a lack of statistical power.
The other investigated cluster of anomalies concerns the production of erroneous responses. As expected, patients with positive symptoms made more perseverations in the free recall task and had a greater bias toward false alarms in the recognition task. The correlation with the bias toward false alarms replicates Bentall and Slade's findings (
11). Similarly, perseverations may also reflect a reality monitoring impairment: the failure to place the response in its appropriate temporal context, that is, in the recall of the appropriate list. Indeed, memory for temporal information has been shown to be impaired in schizophrenic patients (
48,
49). Therefore, this pattern of correlations corroborates Frith's proposition (
17) and other studies linking positive symptoms to a defect in reality monitoring.
On the contrary, positive symptoms were not linked at all to any of the measures of memory efficiency. This could explain why positive symptoms generally fail to show correlations with standard neuropsychological measures: the tests that are commonly used most often measure the efficiency of responses rather than the production of erroneous responses.
On the basis of these results, we may assume that the conflicting reports in the literature concerning the symptomatic correlates of memory could be partly explainable by the different requirements of the task in terms of effortful and automatic processing, as well as differences in the prevalence of depression among the subjects studied. Our results seem congruent with recent findings concerning positron emission tomography studies of brain metabolism. It has been suggested that the left prefrontal cortex is involved in certain verbal memory tasks (
50). Similarly, there is evidence for impairment in left prefrontal cortex metabolism in depressed as well as in schizophrenic patients (
51). Decreased prefrontal metabolism might thus be involved in the mediation of both depressive symptoms and memory deficit in schizophrenic patients. The cognitive dissociation between memory inefficiency and erroneous responses found in the present work may also have cortical metabolic correlates. A dissociation in cerebral metabolism has been observed in schizophrenic patients, with hypoactivity in the prefrontal cortex linked to the poverty syndrome and hyperactivity in the temporal cortex linked to reality distortion (
52).
The two distinct types of deficit suggested by Frith's model were verified by these data, as was the expected link of one with positive symptoms, but not his projected correlations of the other type of deficit with negative symptoms. However, this conclusion concerns only memory tasks. Further studies should explore other cognitive functions in order to see whether severity of depressive symptoms broadly affects cognitive functioning in schizophrenia and whether the previously mentioned dichotomy can be more broadly generalized.