Menopause—the termination of menstrual flow associated with ovarian failure, accompanied by characteristic signs and symptoms (e.g., hot flashes, skin and reproductive tract atrophy, increased body fat) as well as an increased incidence of cardiovascular disease, cognitive impairment, and osteoporosis—is a well-recognized concomitant of aging in women, with an average age at onset of 51.4 years
(1). The endocrinologic, urologic, and geriatric literatures suggest that a possible male equivalent of menopause exists, denoted by such terms as the “male climacteric,” “andropause,” “viropause,” and “low-testosterone syndrome”
(1-
6).
The testes had been linked with sexual vigor and longevity in antiquity; the Greeks and Romans used “satyricon” preparations, made from goat and wolf testicles, as stimulants and aphrodisiacs
(7). Testicular extracts from animals, a form of “organotherapy,” continued to be used into the twentieth century, having been personally endorsed by the French physiologist Brown-Sequard
(7,
8) in 1889, when he claimed rejuvenation and reversal of his own impotence. Further, in the early part of this century, men received testicular implants from animals to reverse the aging process; improvements in energy and cognition were noted, although it is probable that these implants were rejected and that the improvements represented a placebo effect
(7). In the late 1930s, subsequent to the isolation of various androgens
(9), reports were published on treatment of the male climacteric and involutional melancholic symptoms with testosterone. Schmitz
(10) noted improvement in a majority of middle-aged and elderly men with complaints of impotence and depression when they were treated with testosterone propionate, while Werner
(11) noted the relief of fatigue, anxiety, and depression in two men with symptoms of the “climacteric” treated with the same form of testosterone. The problem in these and other reports
(12-
14) on the use of testosterone in men with climacteric symptoms or involutional melancholia was that many reports were of open-label treatment, for different periods of time, of patients who had varying mixtures of signs and symptoms. In addition, not all reports were of positive results
(15,
16), and as noted by Bharke et al.
(9), the American Medical Association’s Council on Pharmacy and Chemistry stated in 1939 that “the involutional melancholia of males, for which testosterone has been suggested, has not been subjected to adequate trials to justify androgenic therapy other than on an experimental basis.” Nevertheless, refinements in the measurement of various hormones (e.g., testosterone, luteinizing hormone [LH], follicle-stimulating hormone [FSH]), and preparations of replacement forms of testosterone have led to an enhanced understanding of the effects of aging on the “well” male, with numerous reports in the nonpsychiatric medical literature on the male climacteric and its treatment
(2–
6,
17,
18). The purported manifestations of the male climacteric include diminished sexual body hair, lowered libido, fatigue, insomnia, hot flashes, anxiety, depression, poor memory, irritability, impotence, and diminished bone and muscle mass
(2–
6,
17,
18).
A MEDLINE search that used the search terms “male climacteric,” “male menopause,” “andropause,” “viropause,” “low-testosterone syndrome,” and “testosterone replacement therapy” was conducted to identify English-language reports on testosterone and aging. This article reviews the literature on the age-associated decline of testosterone in healthy men, starting with an overview of the endocrinology of the normal aging process and the effects of such changes on reproductive, psychosexual, and cognitive functions and muscle and bone mass and continuing with studies on the impact of testosterone replacement on these parameters and the potential adverse short- and long-term effects of such replacement. The clinical issues raised for the field of psychiatry by the literature on the decline of testosterone levels in men are also discussed.
THE HYPOTHALAMIC-PITUITARY-GONADAL AXIS AND AGING IN MEN
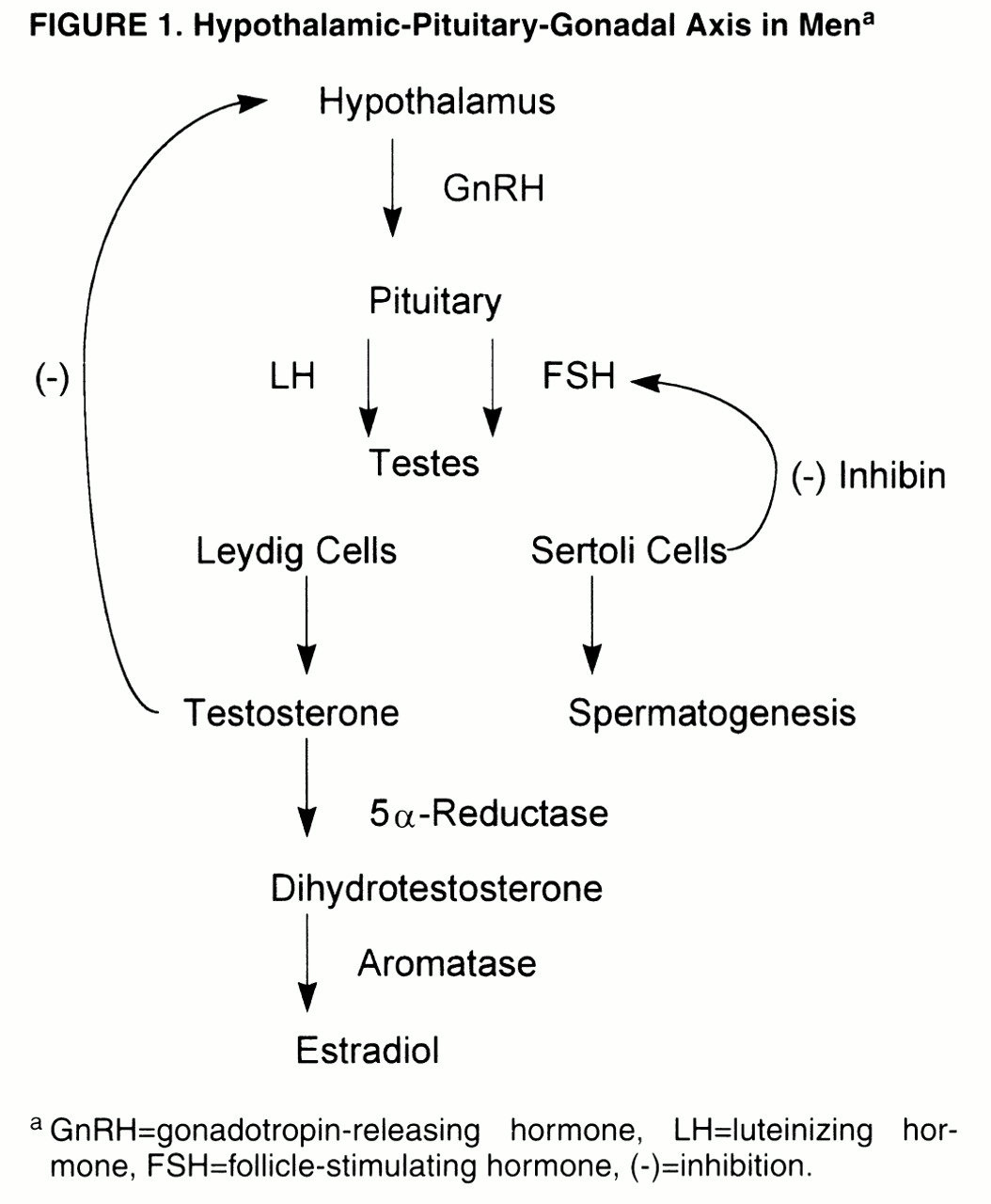
Normal male reproductive function depends on the intermittent secretion of LH and FSH by the pituitary gland under the influence of gonadotropin-releasing hormone (GnRH), which is itself secreted by the hypothalamic GnRH “pulse generator”
(19) (
figure 1). LH stimulates the testicular Leydig cells to secrete testosterone, in a pulsatile manner and in a diurnal rhythm of peak levels in the morning and nadir in the evening
(20), with a negative feedback loop to the hypothalamus to modulate LH secretion. In normal men, approximately 2% of testosterone is in the “free” (i.e., unbound) form, while the remainder is bound to sex hormone-binding globulin (SHBG) and, to a lesser extent, albumin- and cortisol-binding globulin
(17). The term “bioavailabletestosterone” refers to the testosterone that is not bound to SHBG
(5), that is, the free and albumin-bound portion. Testosterone is then metabolized by 5α-reductase, in target organs, to the more potent androgen dihydrotestosterone, which itself is metabolized by aromatase to estradiol
(17). FSH stimulates the testicular Sertoli cells/seminiferous tubules and promotes spermatogenesis; a nonsteroidal Sertoli cell compound, inhibin, in turn regulates FSH level by an inhibitory feedback loop to the pituitary gland and possibly the hypothalamus
(20).
Measurement of serum testosterone is subject to several methodological problems. Rabkin et al.
(21) discussed the variability in the ranges of normal values among laboratories, a problem recently examined by Boots et al.
(22), who reported that the measurement of testosterone by commercially available kits may have limited clinical utility because of the high degree of variability among the kits. Rabkin et al. also noted the wide range of normal values (270 ng/dl to 1100 ng/dl), which indicates that a substantial percent decline in testosterone level could still fall within the normal range, and the variability they found between levels in the same patient on separate occasions even when the time of day for sampling was kept constant.
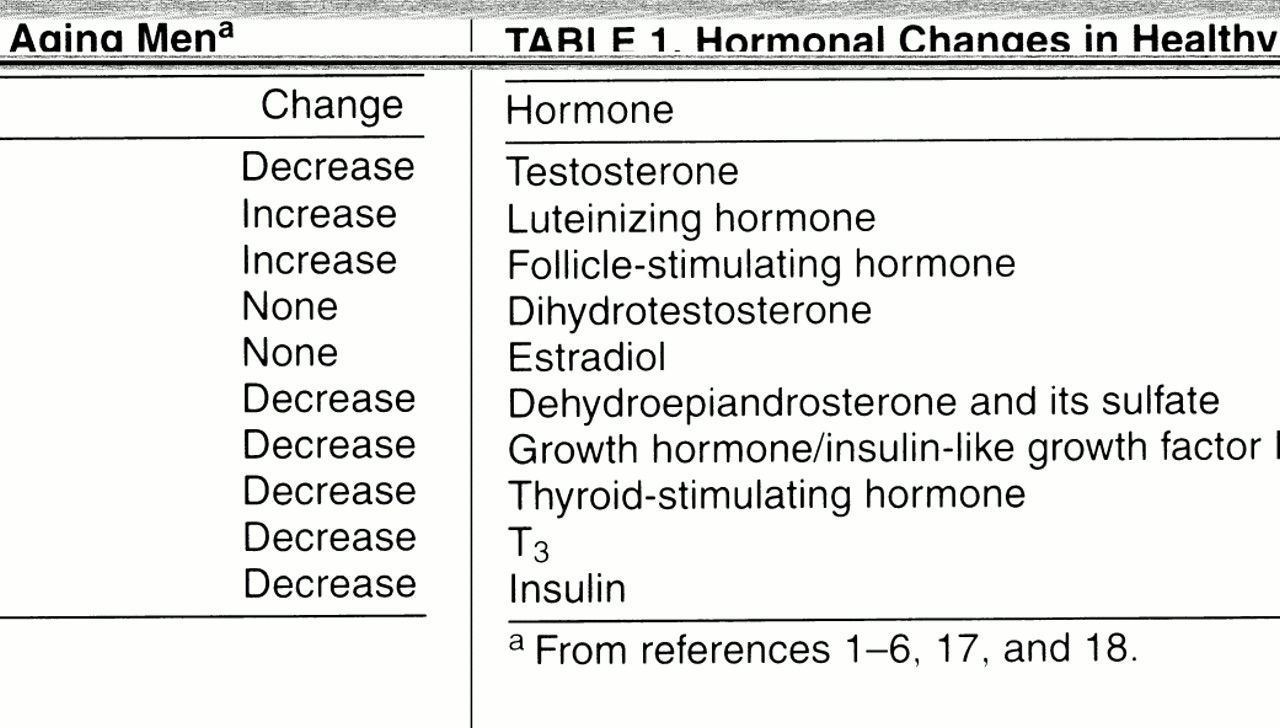
Aging has been associated with a number of changes in the levels of the above-mentioned hormones (
table 1) and, consequently, in the target organs to which these hormones attach. As noted in several reports
(1-
6), testosterone levels in healthy, aging groups of men decline with age on the order of 100 ng/dl per decade
(23), accompanied by increases in SHBG and therefore leading to a decline in free and bioavailable testosterone levels. In addition, LH levels increase (more markedly after age 70), while FSH levels increase and inhibin levels decrease, with a loss of the diurnal rhythm of testosterone secretion
(2-
6). These hormonal changes begin in the 40s and are well established by the age of 50 years. However, there is great interindividual variability in testosterone levels among healthy older men, and therefore, unlike women, not all older men will become hypogonadal as they age
(2). As noted by Tenover
(2), the definition of an older male as hypogonadal has not been established, and the percentage of men described as testosterone deficient depends on what measures are used as cutoff values. Tenover cited, as an example, a 20% rate of hypogonadism if total testosterone levels in men 55 years old with values below the range of normal for young adult men were used, while the rate would reach as high as 50% if bioavailable testosterone levels were used. Levels of dihydrotestosterone do not show a decrease with age, possibly reflecting an increased production by the prostate
(4,
6), and levels of estrogen are also not changed in elderly men
(6).
Testicular failure, due to a decline in Leydig cell mass, is thought not to be the only reason for the hormonal changes described above
(4-
6). The increase in LH levels, for example, is relatively small, except for men older than 70 years, and should be higher in middle-aged and “young old” men if a primary testosterone deficiency were the problem
(4,
5). It is believed that the relatively small increase in LH levels, along with a decrease in relative responsiveness of LH and FSH to GnRH, in elderly men
(4,
6) is more consistent with a failure of the hypothalamic-pituitary system and that the decline in testosterone levels, therefore, represents secondary hypogonadism
(4-
6). Also, since LH secretion is inhibited by endogenous opioid peptides
(24), it has been hypothesized
(5) that increased opioid tone in middle-aged men may keep LH levels from rising, a theory supported by the increase in LH and testosterone levels in hypogondal men treated with the opiate antagonist nalmefene
(25). Finally, a potential change in androgen receptor site sensitivity with aging has been suggested by Bancroft
(26), and aging is associated with a down-regulation of androgen receptors in the rat prostate
(27) and hepatocytes
(28), leading to androgen resistance during senescence.
OTHER HORMONAL CHANGES WITH AGING
In addition to the changes in the hypothalamic-pituitary-gonadal axis, other important changes in endocrine activity take place. As reviewed by Lamberts et al.
(1), two clinically important changes occur in the pancreas and thyroid. Impaired glucose tolerance or diabetes mellitus affects approximately 40% of people between the ages of 65 and 74 years, and in nearly one-half of these it is undiagnosed
(1,
29). This deterioration in glucose tolerance is attributed to several factors, including decreased beta cell secretion of insulin, peripheral insulin resistance, poor diet, increased abdominal fat mass, and physical inactivity
(1,
30). Decrease in pituitary release of thyrotropin (TSH) and decreased peripheral conversion of T
4 to T
3 result in a slight decrease in T
3 levels, which still remain within the range of normal limits. Although diet, oral hypoglycemics, insulin, and exercise are all aspects of managing impaired glucose tolerance, it is not known whether healthy aging individuals would benefit from T
3 supplementation
(1,
31).
Additional endocrine changes occur in the growth hormone (GH)/insulin-like growth factor I (IGF-I) system
(1,
32), with a gradual decline in the pulse amplitude, duration, and fraction of GH secreted, in parallel with a drop in IGF-I levels. These declines, which have been called “somatopause,” are thought to be secondary to a decline in a hypothalamic triggering pacemaker
(1). In addition, age-related declines in the adrenal hormones dehydroepiandrosterone (DHEA) and its sulfate (DHEAS), termed “adrenopause,” appear related to a reduction in adrenal cortical zona reticularis cells
(33,
34). DHEA is a precursor for the formation of estrogenic and androgenic steroids in peripheral tissues
(1).
TREATMENT OF TESTOSTERONE DEFICIENCY
Lamberts et al.
(1) pointed out that there are very few long-term, placebo-controlled studies of testosterone replacement in elderly men. While there does not seem to be disagreement about testosterone replacement in men with low levels and sexual dysfunction, the problems in diagnosing the hypogonadal state
(2), the resultant uncertainty about treatment for men with complaints of sexual dysfunction and low-normal levels of testosterone, and the potential adverse effects of treatment make definitive recommendations problematic. Bhasin and Bremner
(77) suggested testosterone therapy for men with bioavailable levels less than 70 ng/dl, especially if associated with sexual dysfunction. Blood levels are best sampled in the morning and, if low, warrant further evaluation with measurement of LH, FSH, and prolactin levels
(3). If normal free and/or bioavailable testosteronelevels are found, evaluation should be directed toward detecting other causes for the complaints.
Several forms of testosterone replacement are available: oral, injectable, transdermal patches (genital and nongenital), and implantable pellets. Testosterone is well absorbed after oral administration, but rapid hepatic degradation makes it difficult to achieve sustained plasma levels
(15,
77). Also, while the oral 17-alpha-alkylated derivatives of testosterone are relatively resistant to hepatic degradation, their potential hepatotoxicity leaves them unsuitable for clinical use
(77). Parenteral formulations of testosterone are the byproduct of esterification of the testosterone molecule at the 17-beta-hydroxy position. This esterification leads to an increase in hydrophobicity and extends the duration of action
(77), with the longer side chains of testosterone enanthate and cypionate conferring a longer duration of action than the propionate form. The usual dosing of these parenteral formulations is 200–400 mg every 2–4 weeks, but because there is a rapid rise of serum testosterone levels into the high-normal or supraphysiologic range within 24 hours and then a decline into the hypogonadal range over the next 2 weeks, fluctuations in mood, libido, sexual activity, and energy level may occur accordingly. Transdermal preparations, available in scrotal and nonscrotal delivery systems, produce more physiologic levels than the parenteral forms and can mimic the diurnal rhythm of testosterone production
(3,
77). A potential disadvantage of the scrotal system is production of supraphysiologic levels of dihydrotestosterone due to high scrotal 5α-reductase activity; the long-term clinical significance of elevated dihydrotestosterone levels is unknown
(77). The nonscrotal patch, however, is associated with more skin irritation at the application site. Testosterone pellets, which have been available for more than 40 years
(77), are implanted under the skin and release testosterone slowly over 3 months. Because of the need for a skin incision and occasional spontaneous extrusion, this formulation is rarely used.
Reported side effects due to testosterone therapy include increase in hematocrit, gynecomastia, water retention, irritability, acne, hair loss, testicular atrophy, decreased ejaculate, and worsening of sleep apnea
(2–
6,
17,
21,
77). The increase in hematocrit may occur as a result of an effect on erythropoietin or a direct effect on bone marrow stem cells; it was found in up to 25% of patients in one study and is potentially more significant in men with a smoking history, chronic obstructive lung disease, or sleep apnea
(5,
6,
77). Platelet counts and aggregation have also been reported to increase
(3), such that these changes, along with increases in hematocrit, raise concerns regarding adverse cerebrovascular and cardiovascular events.
Gynecomastia, which is believed to result from testosterone conversion to estradiol, leading to growth of breast tissue, is usually transient. Of great concern is the potential effect of testosterone therapy on the prostate and lipid metabolism.
Prostate cancer, which can occur in preclinical (microscopic) or clinical forms, is one of the most commonly diagnosed cancers in the United States; 50% of men have preclinical cancer by their seventh decade
(6). There are no clear data indicating that testosterone therapy will enhance the progression from the preclinical to the clinical form, although it is known that androgens stimulate the growth of clinically diagnosed prostate cancer
(78). Benign prostatic hypertrophy is known to benefit from a reduction in testosterone and dihydrotestosterone, but there is no evidence that replacement therapy will lead to the development of prostatic hyperplasia
(6). The effect of testosterone on lipids is variable. When testosterone esters are given to hypogonadal or eugonadal men, small decreases in HDL cholesterol are seen, while small decreases or no changes are seen in LDL cholesterol, and this differs from the effects of 17-alpha-alkylated androgens, which increase LDL and lower HDL cholesterol and apolipoprotein A-I and A-II levels
(2,
3,
6,
77).
Rabkin et al.
(21) noted that in their studies of testosterone administration to HIV-positive men, an increase in self-reported irritability was the most common side effect, although for the majority of their patients, the effect was transient and “seldom a cause for concern to the patient.” Further, Rubinow and Schmidt
(65) reviewed the relationships between androgens, the brain, and behavior and found that studies of testosterone administration to hypogonadal and eugonadal men did not report increased aggression, although most studies preferentially sampled mood rather than aggression per se. One study
(79) found a decrease in anger in hypogonadal men who had been significantly more angry at baseline compared with control subjects.
Hair loss, secondary to the conversion of testosterone to dihydrotestosterone, was found in 6% of the HIV-positive men treated by Rabkin et al.
(21). They also found acne in 8% of their subjects and decreased testicular size with diminished ejaculate in up to 20%, a side effect they reported treating successfully with human chorionic gonadotropin.
Given the potential for the adverse effects of testosterone described above, a number of reports
(2,
3,
6,
17,
77) noted that the routine use of testosterone therapy should not be recommended unless hypogonadism exists; that is, testosterone therapy is not a remedy for aging in men in the absence of diminished levels of free or bioavailable testosterone.
Lamberts et al.
(1) discussed the sparse literature on the use of DHEA or GH to treat adrenopause and somatopause, respectively, noting that it is not known whether the increase in sex steroid levels induced by DHEA is safe with respect to the development of prostate, ovarian, or other cancers, while the safety of administering GH and thereby elevating GH and IGF-I levels with respect to tumor formation is also unknown, since most human solid tumors express IGF-I receptors. (See reference 80 for a report on the role of DHEA and DHEAS in breast cancer and reference 81 regarding the positive association between IGF-I levels and prostate cancer.)
CLINICAL ISSUES FOR PSYCHIATRY
The concept of a testosterone deficiency syndrome introduces into psychiatry an additional consideration in the evaluation of middle-aged and older men for mood, anxiety, and cognitive disorders. A checklist for the “low-testosterone syndrome” (appendix 1)
(5), with 10 screening questions, reveals considerable overlap with symptoms of primary psychiatric disorders, leaving the psychiatrist with the dilemma of how to distinguish hypogonadism requiring testosterone replacement from primary and other secondary mood, anxiety, and cognitive disorders without measurement of serum testosterone levels. Should all middle-aged and elderly men with mood, anxiety, and cognitive complaints now have routine measurement of serum free or bioavailable testosterone levels when their complaints include sexual dysfunction and/or anergia? Such an undertaking would be both prohibitively expensive and impractical in the context of managed care medicine. Further,as reviewed by Morley et al.
(5), sexual desire was associated with both poor sensitivity and poor positive predictive value regarding low testosterone levels.
Given the complexity of the aging process and the lack of specificity of complaints such as low libido, depression, and anergia, psychiatrists must use their best clinical judgment when assessing middle-aged and elderly men who present with such symptoms. This must include a comprehensive psychosocial, sexual, medical, substance use, and medication history to narrow down the etiology of the patient’s complaints. Serum testosterone measurements should be considered when patients’ complaints cannot be attributed to other causes and in instances where symptoms are only partially responsive or are refractory to conventional psychiatric treatments. Referral to an endocrinologist or urologist should be made when one determines that the patient has a low serum free or bioavailable testosterone level. Education about the normal aging process as well as psychiatric interventions aimed at dealing with associated life transitions and concerns should be part of the treatment plan. In instances in which a patient who comes for psychiatric consultation is already receiving testosterone replacement therapy, it is important to review the circumstances that led to this intervention, including baseline testosterone level, response to treatment, and for those using parenteral preparations, any variations in symptoms that may coincide with fluctuations in serum levels. Finally, given the availability of DHEA as an over-the-counter preparation, patients should be queried about the use of this agent and dissuaded from self-medicating with it, given the unknown long-term risks.