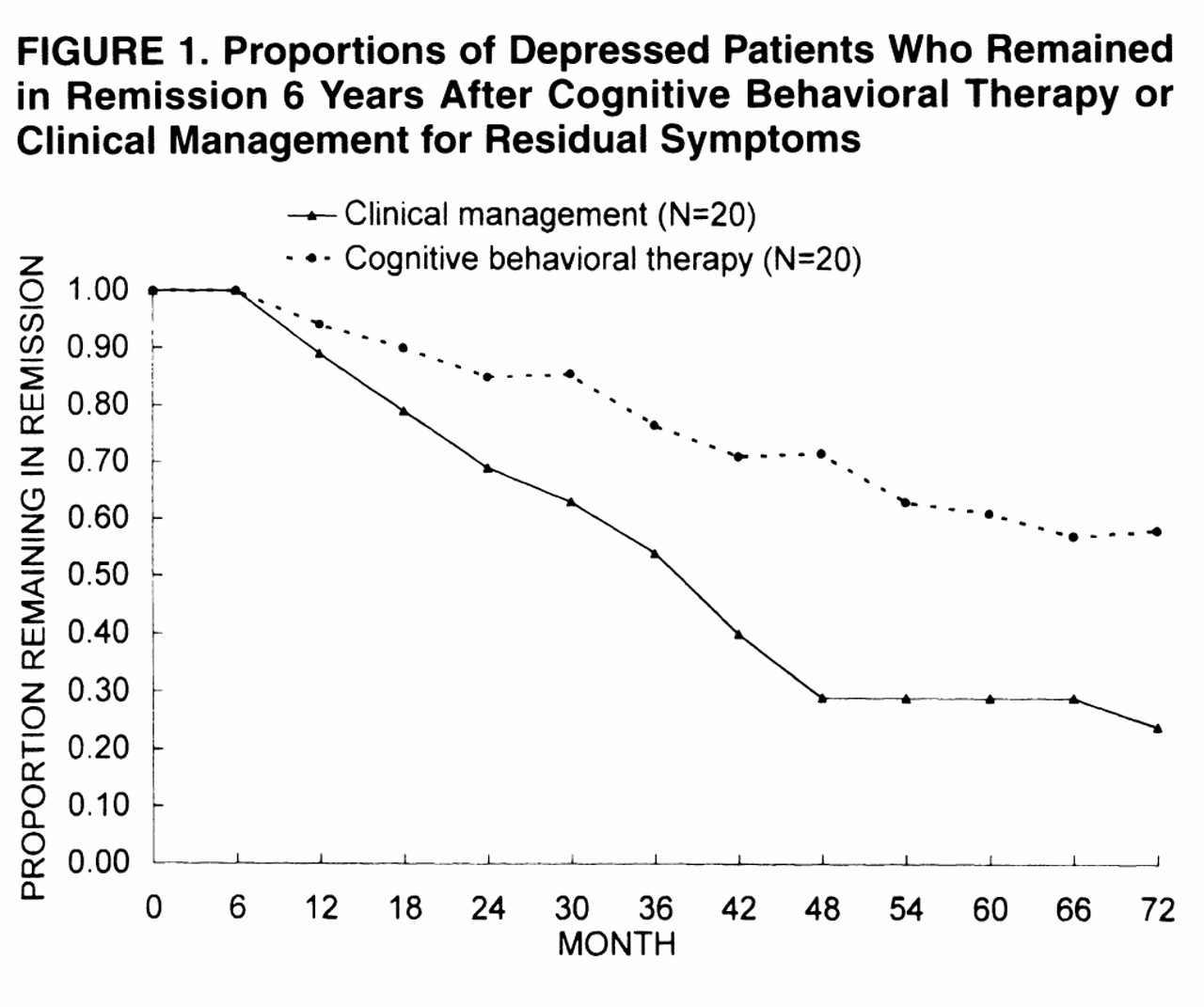
The 4-year follow-up disclosed that cognitive behavioral treatment of residual symptoms of depression has a substantial effect on relapse rate
(3). Such a protective effect for the occurrence of a new depressive episode faded at 6-year follow-up. However, when multiple relapses during the 6-year period were taken into account, patients in the cognitive behavioral treatment group had significantly fewer new episodes of depression than those in the clinical management group. The results thus suggest that treatment of residual symptoms entails a significantly lower risk of relapse within 4 years from the index episode and is associated with an overall more favorable course. The results provide further support to the stage-oriented
(10) model of psychotherapy of depression
(2). According to this model, the psychotherapeutic intervention, which is considerably different from commonly used approaches
(2), is deferred until after pharmacotherapy. The fact that most of the residual symptoms of depression are also prodromal
(2) and that prodromal symptoms of relapse tend to mirror those of the initial episode
(5) provides an explanation for the protective effect of this targeted treatment. Cognitive behavioral treatment may act on those residual symptoms of major depression that progress to become prodromal symptoms of relapse
(11). The methodological limitations of this preliminary investigation, such as the fact that all treatment was carried out by one experienced clinician and our patient group was carefully selected, have been discussed in detail
(2,
3).
Two treatment strategies (maintenance drug treatment and intermittent use of medication with frequent follow-ups and attention to prodromal symptoms) have been outlined in recurrent depression
(11). A potential negative aspect of the latter strategy is concerned with the issue of resistance: the possibility of the patients’ illness becoming refractory to a previously effective treatment by its intermittent use
(12,
13), as reported with lithium prophylaxis
(12). Our results indicate that resistance after rechallenge with a drug that was previously successful also occurs with antidepressant drugs, although only in a small percentage of instances (4%). Further, the possibility cannot be excluded that the two patients in whom resistance occurred were initially placebo responders
(14) and that after they experienced a relapse, their expectations for a second medication trial might not have been as high as for the initial treatment. Fading or tolerance to ongoing antidepressant treatment, however, may also occur
(13) and indeed took place in one patient. The large majority of patients displayed a satisfactory response to drug therapy when a new episode of depression ensued. This confirms the advantages of early treatment of a depressive episode and the value of educating patients to watch for prodromal symptoms of relapse
(11).