Although panic attacks constitute an essential feature of panic disorder, the presence of other psychiatric symptoms is common, including phobic avoidance, anxiety, depression, and ruminations/obsessions, as well as other psychiatric diagnoses, such as depression
(1, 4). Improvement in panic attacks and the closely related symptoms that constitute the standard diagnostic criteria for panic disorder accounts for less than half of the overall improvement associated with recovery from panic disorder
(3), suggesting that additional factors are also important components of the illness.
Assessment of panic attack frequency is difficult, poorly reflects the severity of illness
(5), and is less sensitive to clinical change than other variables used to measure outcome in panic disorder
(6). Despite this evidence and a growing consensus about the importance of a wider spectrum of symptoms
(7), previous trials in panic disorder have generally neglected these components and focused on panic attack frequency as the principal efficacy measure. As a result, it seems likely that such treatment studies have not adequately characterized the effects of pharmacological treatments on panic disorder.
Preclinical and clinical data suggest that brain serotonin systems play a role in the pathophysiology of panic disorder
(8), directly or through modulation of other central systems involved in the regulation of arousal and anxiety
(9). Although tricyclic antidepressants and benzodiazepines have been the mainstay of treatment in panic disorder, evidence suggests that selective serotonin reuptake inhibitors (SSRIs) are equally effective
(10, 11). In the light of the higher rate of adverse events associated with tricyclic antidepressants and the potential for dependency associated with benzodiazepines, SSRIs are likely to become first-line agents for this disorder.
SSRIs have specific antidepressant and anxiolytic actions that are sustained over time. It is likely that these actions contribute to efficacy in panic disorder, providing improved clinical outcomes not only by reducing the frequency of panic attacks but also by improving overall anxiety, depression, phobic symptoms, and measures of social impairment. We hypothesized that the SSRI fluoxetine would be an effective agent in the treatment of panic disorder and that improvement would be better reflected by assessment of symptom domains other than panic attack frequency. The current study was designed to evaluate the efficacy of fluoxetine in the treatment of panic disorder prospectively across a broad spectrum of symptoms in a large, placebo-controlled, randomized, double-blind trial. It was also designed to examine the contribution of different symptom domains to overall improvement during treatment.
RESULTS
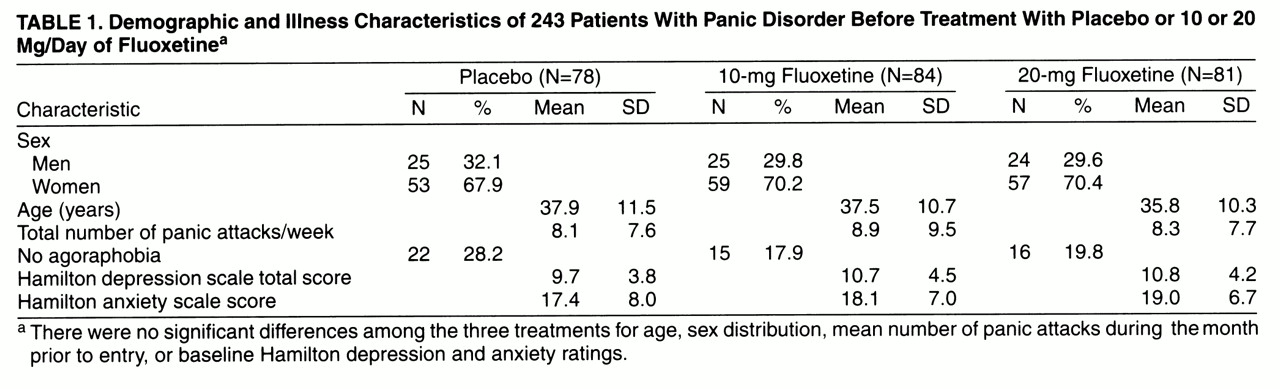
Patient Demographics and Illness Characteristics
Of 364 patients screened by referral and advertisement, 243 patients (169 women and 74 men; mean age=37.1 years, SD=10.8) were randomly assigned to treatment with 10 mg/day of fluoxetine, 20 mg/day of fluoxetine, or placebo. The number of randomly assigned patients per investigator varied from two to 98; three investigators randomly assigned fewer than 10 patients and were pooled for statistical analysis. There were no significant differences among the three treatments for age, sex distribution, mean number of panic attacks during the month prior to study entry, or baseline Hamilton depression and anxiety scores (
table 1).
Acute Treatment Phase
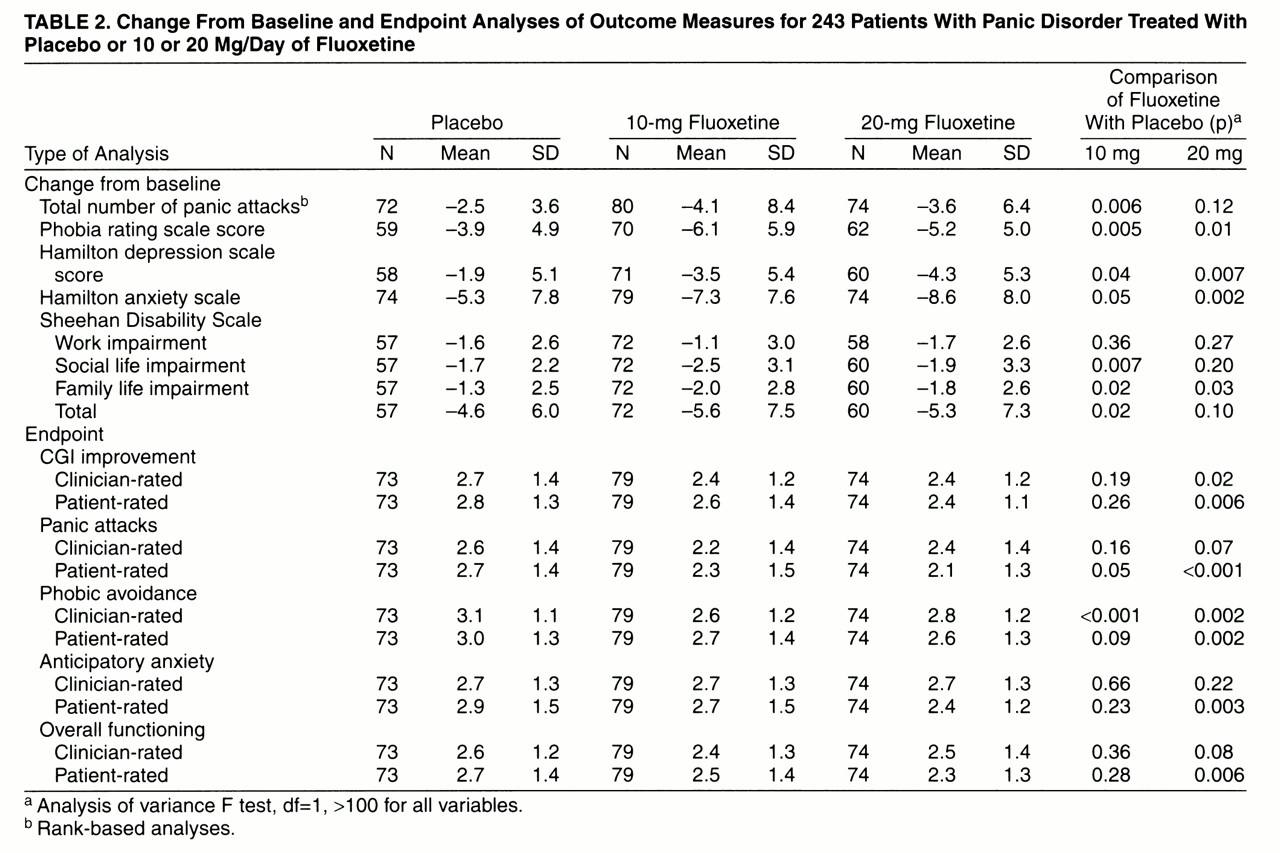
Acute treatment outcomes are summarized in
table 2. Overall response to treatment, assessed by global CGI improvement scores, was statistically significantly greater for patients treated with 20 mg/day of fluoxetine (but not 10 mg/day of fluoxetine) than for those given placebo. The comparison of placebo and combined fluoxetine treatments also favored fluoxetine (F=4.26, df=1, 202, p=0.04).
Patients treated with 10 mg/day of fluoxetine experienced a significantly greater reduction in total panic attack frequency (attacks/week) than did those given placebo (F=7.64, df=1, 202, p=0.006), and a statistically significant difference between the 10-mg fluoxetine group and the placebo group was first observed at week 4 in the last-observation-carried-forward visit-wise analysis. Reductions in total panic attack frequency were not statistically significantly different between the 20-mg fluoxetine group and the placebo group. The comparison of placebo and combined fluoxetine treatments favored fluoxetine treatment (F=6.08, df=1, 202, p=0.02). Among patients who completed the acute treatment phase, reductions in total panic attack frequency from baseline to endpoint were significantly greater in patients treated with 10 mg/day of fluoxetine (F=5.55, df=1, 147, p=0.02) and 20 mg/day of fluoxetine (F=4.48, df=1, 147, p=0.04) than in patients given placebo. Reductions in panic attack frequency were not significantly associated with baseline Hamilton depression scale scores. The three groups did not differ statistically significantly in the number of patients who were completely panic-free at the last visit of the acute phase: 18 (22.5%) of the 80 patients in the 10-mg fluoxetine group; 13 (17.6%) of the 74 patients in the 20-mg fluoxetine group; 11 (15.3%) of the 72 patients given placebo. The groups also did not differ significantly in the number of patients who were free of full panic attacks (four or more symptoms): 37 (46.3%) of the 10-mg fluoxetine group; 32 (43.2%) of the 20-mg fluoxetine group; 27 (37.5%) of the placebo group.
Anxiety, as assessed by Hamilton anxiety scale scores, was statistically significantly reduced in patients treated with 20 mg/day (but not 10 mg) of fluoxetine compared with those given placebo (
table 2). Over the course of the trial, more placebo-treated patients (N=9 [12.2%] of 74) experienced worsened anxiety (defined for a post hoc analysis as increases of 5 or more points in Hamilton anxiety scale score) than did 20-mg-fluoxetine-treated patients (N=2 [2.7%] of 74) (χ
2=4.81, df=1, exact p=0.06) and 10-mg-fluoxetine-treated patients (N=3 [3.8%] of 79) (χ
2=3.70, df=1, exact p=0.07), although the differences were not statistically significant.
Depressive symptoms, as assessed by Hamilton depression scale scores, were statistically significantly reduced in both fluoxetine groups compared with placebo-treated patients.
Clinician-rated phobic avoidance on the Panic and Phobic Disorder Change Scale showed statistically significant improvement in the combined group of patients given fluoxetine compared with those given placebo. Clinician-rated improvement in anticipatory anxiety and overall functioning was not statistically significantly different between either of the fluoxetine treatment groups and the placebo group. Functional impairment, as assessed by the Sheehan Disability Scale, was statistically significantly more improved on both the family life (10-mg and 20-mg fluoxetine groups) and social life (10-mg fluoxetine group only) subscales in the fluoxetine groups than in the placebo group.
Among all patients with a CGI improvement score of 1 or 2 at the end of the acute phase, reductions in panic attack frequency were significantly greater among patients given either dose of fluoxetine than they were among the patients given placebo: among 51 patients given 10 mg/day of fluoxetine, mean frequency=–5.29; among 41 patients given 20 mg/day of fluoxetine, mean frequency=–5.49; among 33 patients given placebo, mean frequency=–3.51. The reductions in panic attacks were significantly greater in both the 10-mg fluoxetine group (F=4.99, df=1, 115, p=0.03) and the 20-mg fluoxetine group (F=5.15, df=1, 115, p=0.03) than in the placebo group. Reductions in phobia rating, Hamilton anxiety and depression ratings, and Sheehan Disability Scale ratings were similar among all groups.
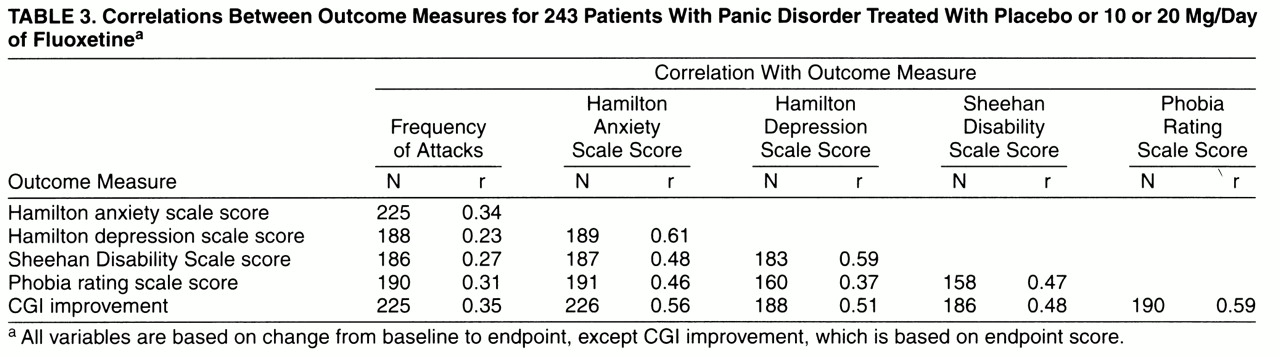
A correlation matrix for clinician-rated outcome measures is shown in
table 3. CGI improvement scores correlated most highly with improvements in phobic symptoms, as well as with reductions in Hamilton anxiety and depression scale scores and Sheehan Disability Scale scores. CGI improvement scores correlated least with reductions in panic attack frequency. Reductions in panic attack frequency correlated poorly with changes in other outcome measures, including Hamilton anxiety and depression scale scores, phobic symptoms, and Sheehan Disability Scale ratings.
Patient-rated overall CGI improvement was statistically significantly greater for 20-mg-fluoxetine-treated patients than for patients given placebo; 20-mg fluoxetine treatment also demonstrated statistically significantly greater improvement in patient-rated panic attacks, anticipatory anxiety, phobic avoidance, and overall functioning. Treatment with 10 mg/day of fluoxetine demonstrated significant improvement in patient-rated panic attacks only. Agreement between patient and clinician ratings on the subscales of the Panic and Phobic Disorder Change Scale was similar for all measures, with correlations on individual items ranging between r=0.71 and r=0.79.
Continuation Treatment Phase
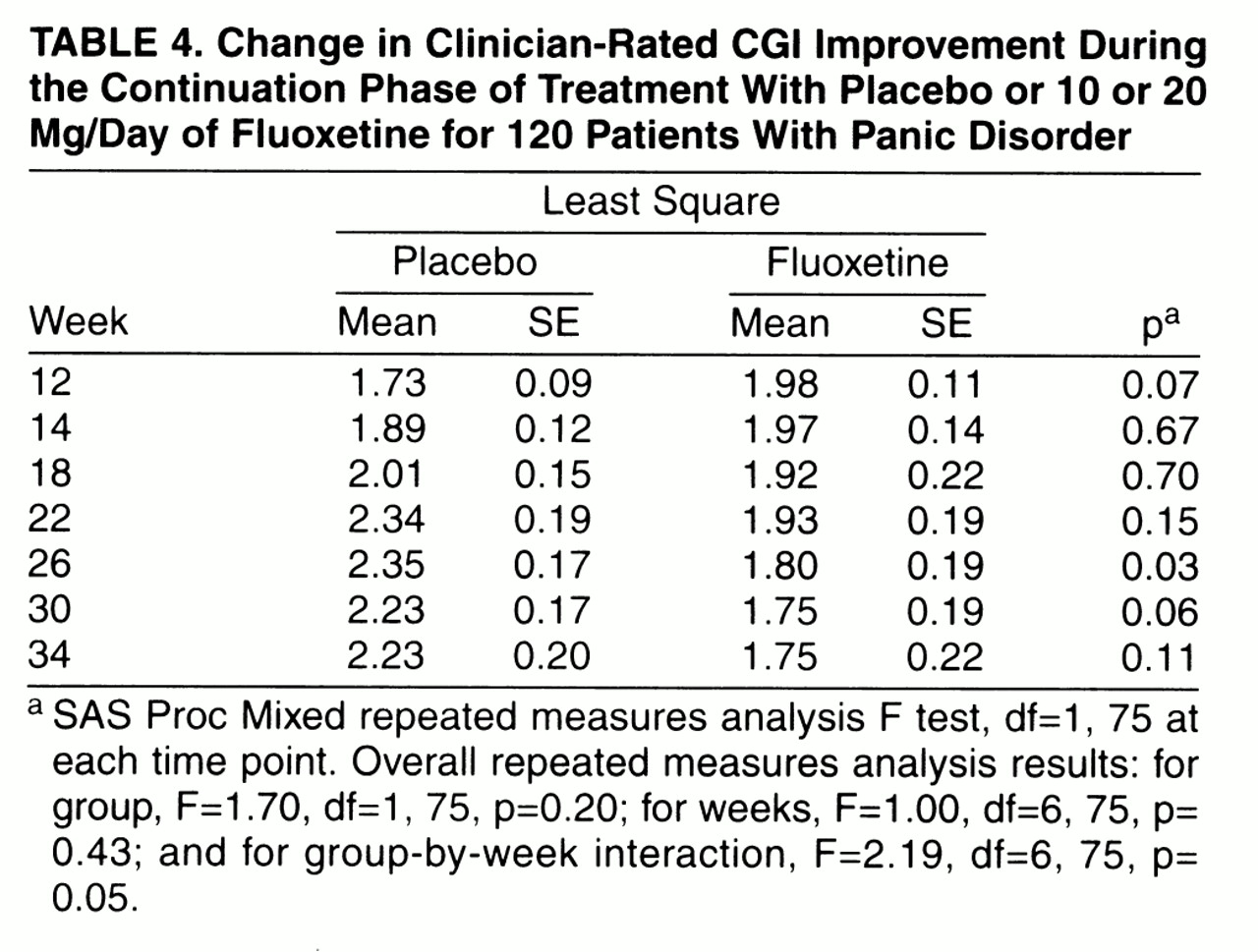
Eighty-eight patients who responded to fluoxetine treatment entered the continuation phase (38 were randomly assigned to continued fluoxetine and 50 to placebo). Additionally, 32 acute-phase placebo responders continued to take placebo. Four patients randomly reassigned to placebo treatment and one patient in the continued fluoxetine group relapsed during continuation treatment. Among acute-phase placebo responders, one patient relapsed during continuation. Repeated measures analysis of CGI improvement scores suggested that more fluoxetine-treated patients experienced continued improvement through the extension phase than patients switched to placebo (
table 4), although the overall tests between groups, between weeks, and the group-by-week interaction were not statistically significant.
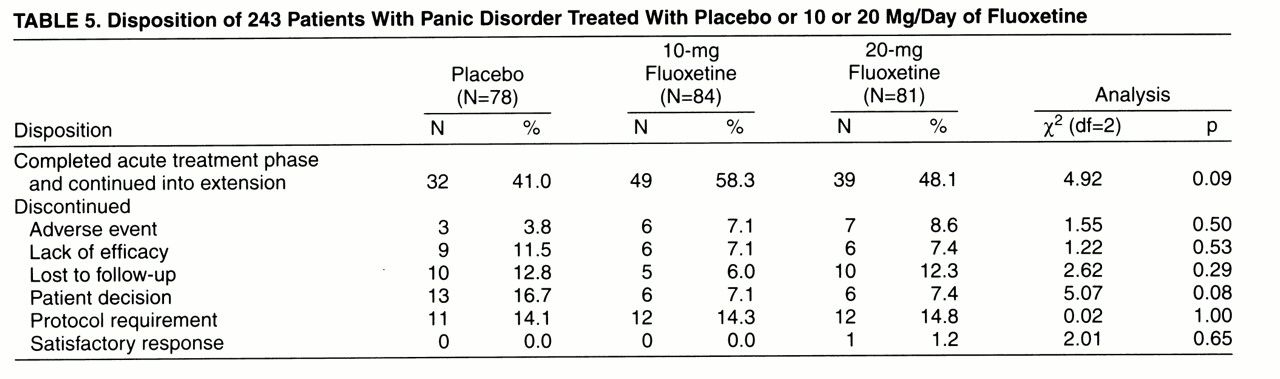
In both phases of the trial, 10-mg and 20-mg fluoxetine treatment was well tolerated. Adverse events were consistent with those observed in clinical trials in other illnesses. There were no statistically significant differences in patient discontinuations due to adverse events between treatments or between pooled fluoxetine treatments and placebo during either acute treatment (
table 5) or continuation treatment (two [5.3%] of the 38 patients who continued to take fluoxetine, four [8.0%] of the 50 who switched to placebo, and two [6.3%] of the 32 acute-phase placebo responders who continued to take placebo discontinued treatment due to adverse events).
DISCUSSION
The results of this study provide evidence for the efficacy and safety of fluoxetine in the acute and continuation treatment of panic disorder and suggest that panic attack frequency is an incomplete measure of clinical response. Treatment with 10 mg/day of fluoxetine was associated with statistically significantly greater reductions in total number of panic attacks than was placebo, and 20 mg/day of fluoxetine, particularly, was associated with statistically significantly greater improvement than placebo in a range of symptom domains, including anxiety, phobia, and depression. This broad response was reflected in superior improvement scores on the CGI and improvement in disease-associated functional impairment. Both 10-mg/day and 20-mg/day fluoxetine doses were well tolerated and had discontinuation rates similar to that of placebo treatment. Correlations between overall improvement and individual symptoms suggested that for all treatments, change in panic attack frequency was less important than changes in other symptom domains as a determinant of recovery.
To our knowledge, this is the first large-scale study of fluoxetine treatment of panic disorder to use a randomized, double-blind, placebo-controlled design. Our results are consistent with previous, uncontrolled reports
(18, 19). The therapeutic effect of fluoxetine treatment appears to be a primary anxiolytic and antipanic action rather than secondary to reduction of depressive symptoms, evidenced by the fact that the decreases in panic frequency were not related to the degree of depressive symptoms present at the start of the study, measured by Hamilton depression scale scores. The time course of response, with efficacy superior to placebo beginning at week 4, is consistent with the antipanic actions of other antidepressants.
Although panic attacks are a core part of the diagnostic criteria for panic disorder, a body of data suggests that much of its associated morbidity and resulting functional impairment result from other symptom domains
(2). In this study, in addition to reducing panic attack frequency, fluoxetine treatment also reduced measures of anxiety, depression, and phobic avoidance. These changes could potentially be individually mediated by effects of fluoxetine on multiple distinct serotonin pathways or, alternatively, could be “downstream” effects related to actions at a particular physiological locus.
Although the use of panic attack frequency as a clinical endpoint is problematic
(20), there have been few systematic assessments of this issue. Consistent with a previous report
(3), the correlations in this study between global and symptom improvement and improvement in functional measures such as anxiety, depression, phobic avoidance, and disability were more robust than correlations between global improvement and reductions in panic attack frequency, suggesting that improvements in these measures were more reflective of clinical improvement. These “secondary” outcome measures were more highly correlated with one another than with reduction in panic attack frequency. These data suggest that change in panic attack frequency does not adequately reflect improvement as a primary measure in psychiatric illness. By contrast, in a previous series of clinical trials assessing the efficacy of fluoxetine in the treatment of depressive illness, Hamilton depression scale score changes correlated much more highly with CGI improvement score changes (r
2 ranged between 0.5 and 0.6) (D. Faries, personal communication) than panic attack frequency change scores did with CGI improvement score changes in the current study (r
2=0.12). These data support the consensus conference findings reported by Shear and Maser
(7) regarding the limitations of panic attacks in assessing treatment effects and suggest that panic attack frequency is of limited clinical relevance as an outcome measure.
The lack of an association between reduction in panic attack frequency and overall recovery is probably related to both methodological issues and clinical factors. None of the options for tracking panic attacks is satisfactory. The use of diaries to report attacks is unreliable
(20), and retrospective counting at fixed intervals invites recall bias. Patients cannot consistently differentiate between full and limited-symptom attacks
(5), creating inconsistencies in what is actually counted as an attack. Finally, panic attack frequency is quite variable, and counts in a given interval may be discordant from more stable measures of improvement. Thus, although the panic attack is thought to be the pathognomonic feature of panic disorder, data from this and previous studies suggest that panic attack frequency is unsatisfactory as a measure of treatment outcome and may not be primarily responsible for disease-associated morbidity. For these reasons, clinical improvement in panic disorder is better reflected by multidimensional measures specific to panic disorder that measure panic attack frequency as one among several important variables, such as the Panic Disorder Severity Scale
(14).
Interestingly, however, this study does provide some evidence supporting the concept of panic attacks as specific symptoms that differentiate this illness from other anxiety disorders. In a post hoc analysis, among fluoxetine- and placebo-treated patients with comparably good overall responses to treatment, both doses of fluoxetine were associated with significantly greater reductions in panic attack frequency than placebo, but reductions in other symptom measures were similar. This suggests the possibility that fluoxetine is associated with specific effects on panic attack frequency beyond the benefits associated with intervention more generally. Reports of dose-response relationships for SSRIs in panic disorder have varied. The reported minimum target dose of paroxetine is 40 mg/day (SmithKline Beecham Pharmaceuticals 1996 prescribing information), sertraline is reported to be effective over a range of 50 to 200 mg/day (Pfizer Inc. 1997 prescribing information), and citalopram is reported to be effective between 20 and 60 mg/day
(10). In the current study, some therapeutic effect was noted in patients treated with 10 mg/day of fluoxetine, including a reduction in panic attack frequency and an improvement in function (measured by the Sheehan Disability Scale), but the clinician-rated CGI improvement score, as well as most patient-rated measures, were not statistically significantly different from placebo. Patients treated with 20 mg/day of fluoxetine had reductions in symptoms across a broader range of measures, including phobic symptoms, anxiety, depression, and measures of functional impairment. Although we did not examine doses above 20 mg, fluoxetine dose-response relationships in nondepressive disorders
(21, 22) suggest that some patients who failed to respond at 20 mg/day could have responded to a higher dose.
Many patients whose illness responds to treatment experience a return of symptoms over time
(23); however, few controlled long-term treatment studies have been published, and the long-term course of panic disorder, including relapse rates after stopping successful treatment, is uncertain. In this study, fluoxetine-associated improvement appears to have continued during the extension phase, and relapses were numerically lower in patients who continued fluoxetine treatment; however, overall rates of relapse were extremely low, and the difference was not statistically significant. We are aware of only one previous report of a study that randomly reassigned acute-treatment responders to continued treatment or placebo
(24; SmithKline Beecham Pharmaceuticals 1996 prescribing information). In it, patients who responded to fixed-dose paroxetine treatment (10, 20, or 40 mg/day) and continued treatment for 3 months were protected against relapse for a further 3 months compared with placebo. However, relapse rates for patients who continued on paroxetine were similar to those observed in our study for patients who continued on fluoxetine (<10%), while relapse rates for patients who discontinued paroxetine were greater than 30%. Inspection of these data suggests that this difference was largely related to high relapse rates (>50%) among patients who required 40 mg/day of paroxetine during the acute phase, since relapse rates for the lower doses were comparable to those observed in our study. The greater relapse among patients receiving 40 mg/day of fluoxetine could reflect the presence of a subgroup of patients with different vulnerability to relapse who responded to the higher dose but not the 10- or 20-mg/day doses. Alternatively, it could reflect “rebound” effects related to discontinuing the higher dose.
Other factors could also account for the low relapse rates. Some patients who chose not to enter the continuation phase or left the study early for reasons other than relapse may have done so for reasons related to symptom return. The definition of relapse used (CGI improvement score ≥4) was relatively insensitive and allowed patients with some recurrent symptoms to remain in the study. Nonetheless, results of the repeated measures analysis of CGI improvement scores suggest that continued treatment with fluoxetine conferred a protective effect not seen in patients switched to placebo. Further, the consistency of the relapse rates on the lower doses of paroxetine and fluoxetine may accurately reflect the course of illness for patients who respond to acute treatment, and it is possible that patients with panic disorder are more likely to experience gradual, mild-to-moderate worsening over a 6-month period than an abrupt return of symptoms.
Fluoxetine treatment was well tolerated in panic disorder. Differences in discontinuations due to adverse events among the fluoxetine and placebo groups were not statistically significant, and fewer fluoxetine-treated patients than placebo-treated patients experienced worsened anxiety as measured by deterioration of Hamilton anxiety scale scores. These data contrast with previous uncontrolled reports that fluoxetine should be started at very low doses and raised very slowly in patients with panic disorder
(18, 25).
In summary, fluoxetine treatment was associated with statistically significantly greater relief than placebo treatment over a broad range of symptoms, including measures of social and family functioning, particularly at a dose of 20 mg/day. The safety profile was favorable and similar to that established in patients with depression. These data provide evidence that fluoxetine is an effective, well-tolerated agent for the treatment of panic disorder. These data further suggest that although reduction in panic attack frequency is a component of fluoxetine’s therapeutic effect, effects on phobic avoidance, anxiety, and depressive symptoms are also critical factors in clinical improvement. Outcome measures that take into account this broader range of symptoms rather than focusing narrowly on panic attack frequency appear to more accurately assess the therapeutic benefit of medical intervention.
ACKNOWLEDGMENTS
The Fluoxetine Panic Disorder Study Group included the following investigators and their staffs: R. Bruce Lydiard, M.D., Ph.D., Mark H. Pollack, M.D., Charles H. Merideth, M.D., Jeffrey S. Simon, M.D., William M. Patterson, M.D., Gregory Asnis, M.D., Dennis Munjack, M.D., Peter Schram, M.D., Jeffrey Apter, M.D., and Lynn Cunningham, M.D.