Tobacco dependence is the most common substance abuse disorder and the leading preventable cause of death in the United States (
1). It meets all of the DSM-IV criteria for drug dependence, including compulsive use, difficulty in quitting, and withdrawal symptoms upon cessation of chronic use. Only about 3% of smokers who try to quit remain abstinent for 1 year (
2). Although there are more than 2,000 compounds in cigarette smoke, nicotine alone has been shown to produce tolerance, dependence, and a distinct withdrawal syndrome in both animals and humans (
3). It is generally considered to be the addictive and reinforcing agent responsible for continued smoking behavior (
1,
4,
5), and while the mechanisms underlying its reinforcing properties are not well understood, nicotine is thought to interact with the mesocorticolimbic dopamine system in a manner similar to that of other abused drugs such as cocaine (
3,
6,
7).
Nicotine produces profound behavioral effects in humans, including memory facilitation, locomotor activation, mild anti-nociception, calming, and appetite suppression (
8). However, little is known of nicotine's effects on neuronal activity at a systems level in animals (
9–
11), and almost nothing is known about this in humans. Understanding the sites and mechanisms of nicotine's action in the human brain, along with our knowledge of its behavioral pharmacology and its potential addictive properties in common with those of other abused substances, may lead to new concepts related to the central mechanisms of drug dependence and the development of novel smoking cessation therapies.
Functional magnetic resonance imaging (MRI) allows for the noninvasive study of human brain activity by measuring localized, intrinsic signal changes that are directly driven by changes in neuronal activity. Because of its excellent temporal and spatial resolution, we used this imaging tool to identify neuroanatomical regions activated by nicotine in the human brain. We hypothesized that frontal lobe and limbic/cingulate cortical structures would be activated by nicotine, consistent with the drug's mood-altering, attentional, and vigilance properties. Further, since nicotine is a stimulant and addictive agent, we hypothesized that regions previously implicated in animal models of drug abuse, such as the nucleus accumbens, would also be activated in humans after nicotine administration.
METHOD
Subjects were recruited through newspaper advertisements. They were generally healthy individuals (nine male and seven female) between the ages of 18 and 39 years (mean=25.9, SD=6.0) with smoking histories averaging 8.5 years (SD=5.4, range=1.5–23) and current use of about one pack of cigarettes per day. All but two subjects were strongly right-handed according to the Edinburgh Handedness Inventory (
12). The subjects had no previous history of any neurological or psychiatric disorder and no other drug dependence. The experiments were approved by the institutional review board of the Medical College of Wisconsin. Brief physical and history examinations were conducted before the initiation of any experimental procedure. After complete description of the study to the subjects, written informed consent was obtained. Subjects were instructed that they should smoke as they wished during all phases of their participation in the study. Their only restriction was no alcohol consumption for 24 hours and no caffeine consumption for 12 hours before scanning.
To determine safety and obtain physiological and behavioral measures, before their scanning session the subjects received an intravenous injection of saline into an antecubital vein, followed by three injections of nicotine (0.75, 1.50, and 2.25 mg/70 kg of body weight). All injections lasted for 1 minute and occurred in ascending dose order every 20–30 minutes while the subjects were recumbent in bed at the Medical College of Wisconsin General Clinical Research Center. Subjects were continuously monitored for blood pressure and pulse rate and by ECG and were asked to respond on 10-point Likert scales at 2, 5, and 15 minutes after injection to eight behavioral rating questions (feelings of “rush,” “high,” pleasantness, anxiety, confusion, and sedation, intensity of drug effect, and drug liking). A second intravenous catheter was used to collect blood samples for determination of serum nicotine concentrations by gas chromatography-mass spectrometry (
13).
Approximately 1 week later, the same three doses of nicotine were administered while the subjects underwent functional MRI scanning. The subjects reported to the MRI facility approximately 30 minutes before their scan appointment. All of them reported smoking a cigarette immediately before entering the hospital. Scan sessions, approximately 2 hours in duration, began between 8:00 p.m. and 1:00 a.m.
Whole brain functional MRI data were acquired with a 1.5-T Signa scanner (GE Medical Systems, Milwaukee) equipped with a 30.5-cm internal diameter three-axis, balanced-torque local gradient coil designed specifically for rapid gradient switching and a shielded-quadrature elliptical encapped transmit/receive birdcage radio frequency coil inserted inside the gradient coil (
14). Eight contiguous 8-mm axial slices were acquired with use of a blipped, gradient-echo, echo-planar image pulse sequence (TE=40 msec) with an interscan resolution of 6 seconds during the 20-minute acquisition period and a 24-cm field of view with an in-plane resolution of 3.75 mm. During each 20-minute scan, the first 4 minutes consisted of baseline data acquisition, followed by the 1-minute intravenous injection, and then 15 minutes of postdrug data acquisition. The blood-oxygen-level-dependent pulse sequence we used is weighted to be most sensitive to changes in blood oxygenation levels rather than blood flow alterations. Anatomical images (spoiled gradient/recall acquisition in the steady state [GRASS], 256×256 pixels, 1.1 mm thick) were acquired immediately before functional MRI data acquisition.
Functional MRI data were analyzed with an algorithm and associated software that we developed, which is based on the working hypothesis that parenchyma-derived signals should follow a pharmacokinetic model; that is, the arterial drug concentration curve is posited to reflect the brain drug concentration distribution, which in turn drives brain activation patterns (A.S. Bloom et al., manuscript submitted for publication). Active voxels were detected according to six criteria: time to peak effect (1–8 minutes after the start of the injection), magnitude and statistical significance of the peak effect (≥0.5% over baseline and p≤10
–6, unpaired t test), slope of the rising phase of the response curve (0.5–5.0), time to decline back to 50% of the peak effect (4–12 minutes after injection), and baseline period stability (≤11% of the mean range for all voxels in the brain). The acceptable range given in parentheses for each parameter was determined from the known pharmacokinetics of nicotine, the observed venous blood levels, and the physiological and behavioral effects seen in this study. To be considered active, a voxel had to meet all of the six criteria. Functional images were generated by applying the algorithm to each pixel and overlaying activated regions on high-resolution, three-dimensional spoiled GRASS images. Nearest-neighbor pixel analysis was performed to preclude isolated pixel recognition. All data were transformed into the stereotaxic coordinate system of Talairach and Tournoux (
15), averaged across all subjects at each dose to produce mean dose-response activation maps, and displayed with the use of AFNI (
16). To compensate for the anatomical uncertainty of the Talairach and Tournoux transformation, a 3-mm blur was formed around activated pixels.
Statistical significance for the combined group data was determined on the basis of a probability function derived from a beta binomial distribution of each individual's response after the saline injection. A centers-of-mass analysis was used to determine regions of interest, with a minimum volume of tissue set at 150 ml. Clusters below this size were ignored in further analysis. The intensity of all pixels within the cluster was set at the maximum intensity within the cluster. Across all subjects, after the saline injection, less than 3% of the voxels in the brain met the strict criteria for activation outlined above. Further, in the group mean activation maps, no clusters of 150 ml or greater were found after the saline injection, indicating a very low probability of a false positive with use of this waveform analysis method.
RESULTS
When measured 2 minutes after administration, nicotine had no significant effect on heart rate or blood pressure. Mean arterial pressure increased only transiently for 10–20 seconds, from a baseline of about 91 mm Hg to a mean peak of about 99.5 mm Hg. Nevertheless, after the nicotine injections the subjects reported experiencing moderate “rush” and “high” feelings that were dose- and time-dependent (mean peak rush scores=2.5, 4.6, and 5.7 out of a possible 10 after the low, medium, and high doses, respectively). Both the rush and the high peaked at 2 minutes after injection and returned to baseline by 5 minutes after. In contrast, scores on pleasantness averaged about 5 at all doses and persisted for 15 minutes. Finally, the participants reported moderately liking the experience (mean peak response rating=4.1, SD=2.1), with no significant effect on feelings of sedation, confusion, or anxiety. Plasma nicotine levels (
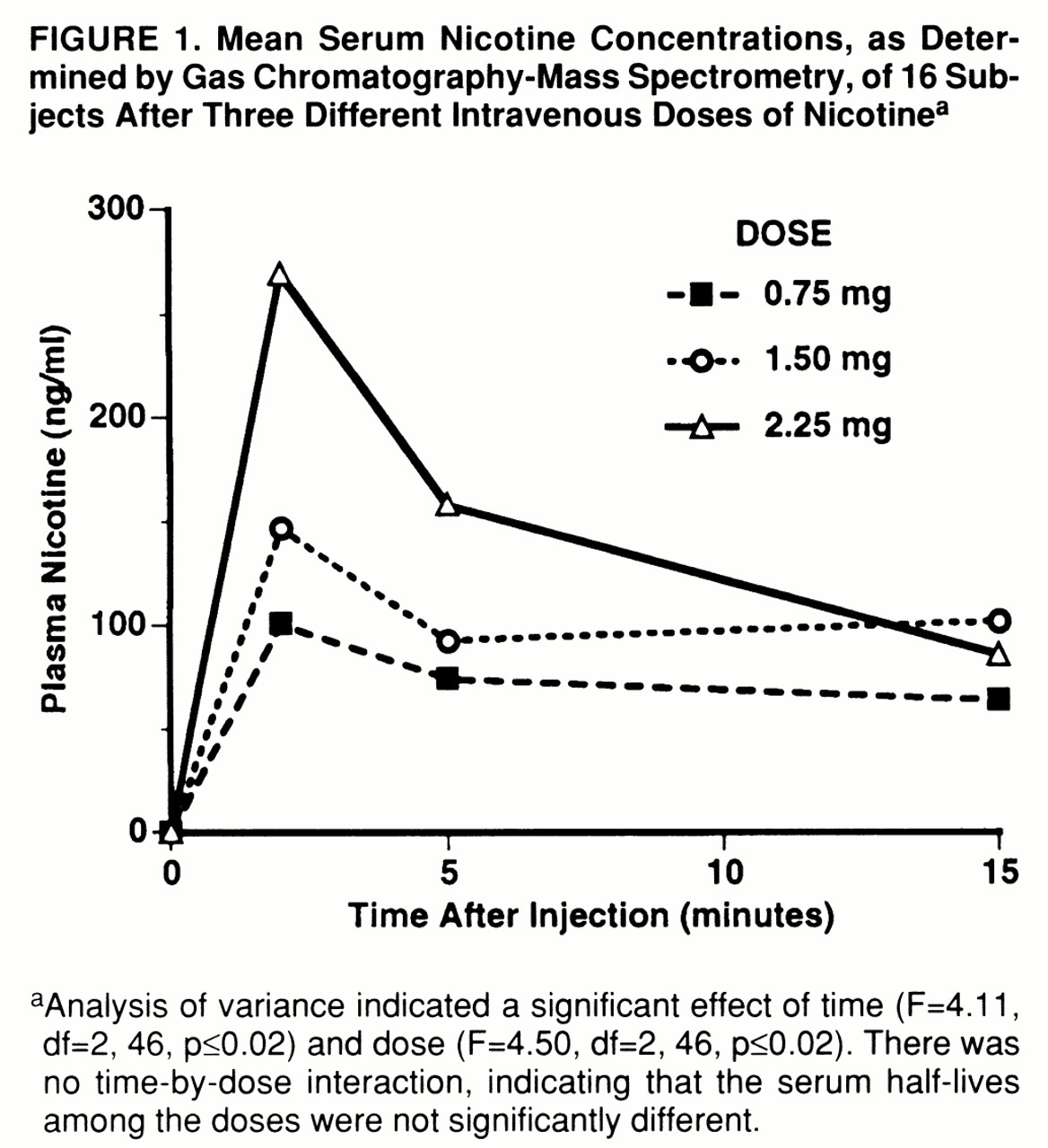
figure 1) accurately approximated both the behavioral and functional MRI signal time courses. Dose-dependent increases in plasma levels reached maximum values at 2 minutes and rapidly decreased to about two-thirds of peak value within 15 minutes. Baseline nicotine levels were virtually undetectable at less than 10 ng/ml. No prolonged untoward effects were ever reported; all subjects agreed to receive all nicotine doses, and all returned for subsequent functional MRI scanning.
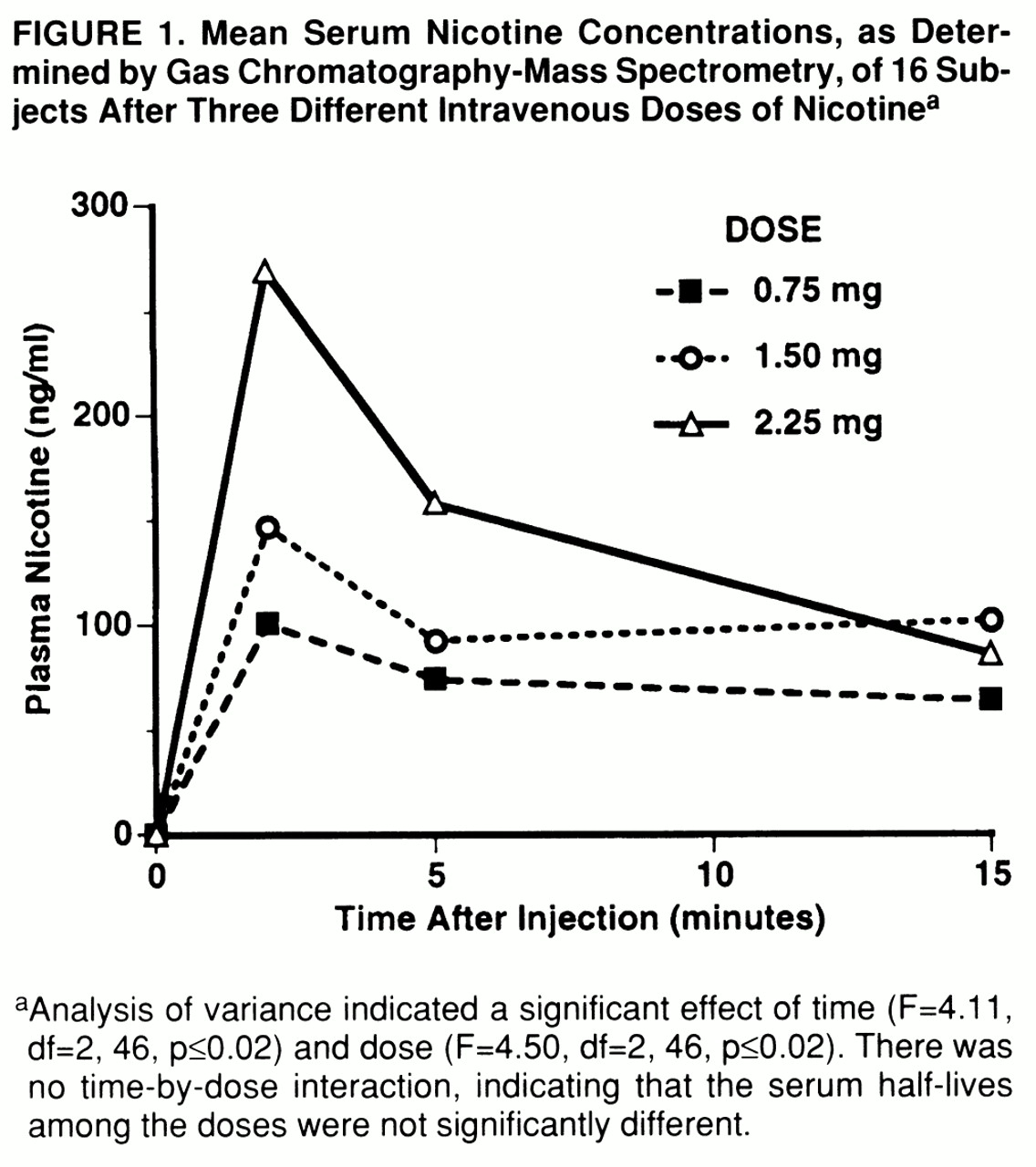
Regional brain functional MRI signal intensity rapidly increased from baseline levels after nicotine administration, reached a peak response approximately 2.8 minutes after the end of the 1-minute intravenous injection, and returned to one-half of the maximum level approximately 5.6 minutes after drug administration (
figure 2). While this response pattern was not the only one seen, it was by far the most dominant and the one used to extract drug-induced localized brain activation from the functional MRI signal. When averaged across all participants, neither percent signal change, time to peak effect, nor time to one-half the maximum effect changed significantly as a function of dose. In contrast, the percentage of voxels activated increased from 3.38% after saline to 7.06%, 11.06%, and 10.90% after the low, medium, and high nicotine doses, respectively (F=14.35, df=3,45, p<0.0001). The numbers of activated voxels were greater after both the medium and high doses of nicotine compared to saline, and the medium dose effect was also greater than the low dose effect (Scheffé F test, p≤0.05).
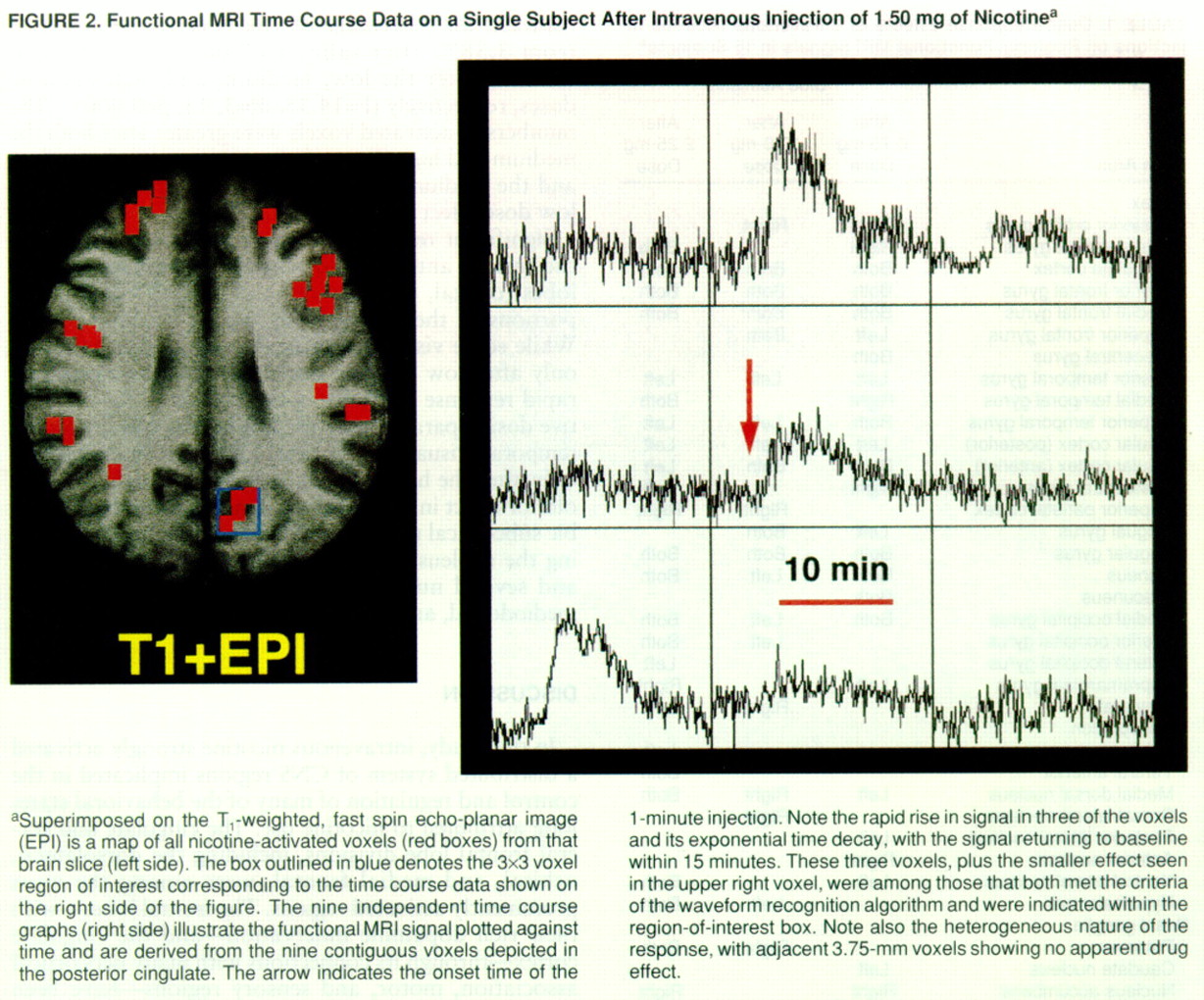
Significant regional activation (
table 1) was seen in the insula, anterior and posterior cingulate, frontal lobes (orbital, dorsolateral, and medial frontal), and portions of the temporal and visual occipital cortex. While some visual and frontal regions were activated only after low nicotine doses, perhaps demonstrating rapid response tolerance in these areas to the cumulative dosing paradigm we used, other regions (including temporal, visual, and parietal lobes) became activated only after the higher doses, suggesting a higher threshold for effect in these regions. Finally, a number of limbic subcortical regions were activated (
figure 3), including the nucleus accumbens, amygdala, hypothalamus, and several nuclei within the limbic thalamus (e.g., mediodorsal, anterior, and lateroposterior nuclei).
DISCUSSION
In this study, intravenous nicotine strongly activated a distributed system of CNS regions implicated in the control and regulation of many of the behavioral states long attributed to nicotine use. The cingulate and several frontal lobe divisions, including the dorsolateral, orbital, and medial frontal, were among the most prominently activated regions. The frontal lobes—with their rich dopamine innervation—and the cingulate cortex—through its connections with many neocortical association, motor, and sensory regions—have been thought to be involved in the processing of such diverse cognitive states as working memory, attention, motivation, mood, and emotion (
17,
18). All of these are known to be modified by nicotine intake (
19). In addition, nicotinic receptors are present on both the somatodendritic and axon terminals of locus ceruleus noradrenergic neurons (
20), which are known to project to much of the forebrain and hippocampus. These locus ceruleus neurons and their projections are thought to regulate or modulate behavioral arousal and vigilance (
21). Taken together with what is known of brain regional structure-function relationships, the functional MRI brain activation pattern in this study is consistent with recurrent reports by cigarette smokers that smoking enhances—whereas smoking abstinence and withdrawal compromise—arousal, mood, vigilance, and attention, among other cognitive processes (
19).
Although cigarette smoking has been reported to modify numerous cognitive behaviors, including attention and working memory (
8,
19), nicotine's behavioral properties in humans have been difficult to attribute to specific brain regions because of the sometimes subtle and diffuse nature of the drug's effect and the rapid smoking-withdrawal cycles that make “baseline” behavioral levels difficult to define. This observation may help explain why the cognition-enhancing properties of nicotine have been most convincingly demonstrated in acutely abstinent smokers. It has been more difficult to demonstrate these properties in nonabstinent smokers and in nonsmokers (
22), suggesting that some of the reported behavioral effects may reflect withdrawal relief. It has not been demonstrated whether nicotine replacement acts in a “nonspecific” fashion to ameliorate withdrawal-induced dysthymia (alleviation of which might be manifested as a positive effect on cognition) or acts directly on CNS structures primarily involved in cognitive systems. However, in view of the brain regions activated in this study, our data support the hypothesis that direct activation of the frontal and cingulate regions by nicotine is responsible for the drug's primary behavioral and mood-altering effects.
Although cigarettes generally produce only a moderate euphorigenic state, human cocaine abusers often identify the effects of intravenous nicotine as similar to and, in many cases, identical to those of intravenous cocaine (
4). This observation suggests that the reinforcing and drug-discriminative properties of both drugs probably share common anatomical sites and mechanisms through their ability to ultimately enhance mesocorticolimbic dopamine transmission, albeit through different cellular mechanisms. In this study, among the regions that nicotine activated were the nucleus accumbens, amygdala, limbic thalamus, and frontal lobe cortical regions that have consistently been implicated in the reinforcing properties of both nicotine and cocaine in animal experiments (
4,
23–
25), suggesting the two drugs' common mechanisms in humans as well. Indeed, the only brain area in the rat that supports direct cocaine self-administration is the medial prefrontal cortex (
26), the human homologue of which includes the mediodorsal thalamus terminal fields of the orbitofrontal and medial frontal lobes (
27)—regions activated by nicotine in the present study.
To our knowledge, there have been no previously published human studies that have used functional MRI to examine regional patterns of neuronal activity after acute nicotine administration. In two preliminary reports (each with three subjects) (
28,
29), Nagata et al. (
28) reported increased cerebellar and frontal lobe blood flow after cigarette smoking in a study that used
15O positron emission tomography (PET), while London (
29) reported a generalized decrease in glucose uptake after 1.5 mg i.v. of nicotine. Another previous indication of the central sites of nicotine action in the human brain came from the distribution of nicotine-binding sites noted with PET (
30). Although receptor binding does not necessarily indicate primary site of action, the present functional activation data agree remarkably well with these human receptor maps. The largest concentrations of [
11C]nicotine binding sites are seen in the frontal, cingulate, and insular lobes of the cortex and in the thalamus and basal ganglia. In addition to our observation of significant functional MRI activity in all of these regions, a notable finding from the present study is the significant activation of the visual cortex. This activation may have been the result of primary nicotinic receptor binding in subcortical visual relay nuclei (e.g., the lateral geniculate) that are known to have high nicotinic receptor concentrations (
31,
32) or presynaptic nicotinic binding in the cortex. Nicotinic receptors have also been reported in many thalamic nuclei in rats (
33). These nuclei project, in turn, to the temporal lobe auditory and visual occipital cortex and widespread regions of the frontal, cingulate, and parietal cortex. Thus, some of the most activated cortical regions after administration of intravenous nicotine in this study may reflect intense subcortical, thalamic nicotinic receptor activation.
The observation of unilateral regional activation following drug administration was unexpected and deserves mention. Several recent human metabolic mapping studies have reported right dominant activation after administration of cocaine (
34) and marijuana (
35). While most of the activation sites reported in
table 1 were bilateral, many cortical and subcortical sites were activated only unilaterally. Left-right differences in drug effects should be evaluated more closely, since they relate to changes in drug-induced behavioral states.
It is unlikely that the changes in functional MRI signal which we observed were the result of peripheral or central cardiovascular effects. Although nicotine can produce changes in heart rate and blood pressure (
3), which by themselves can indirectly alter global cerebral blood flow, no significant cardiovascular changes were seen in the present experiment, which involved wellexperienced, nicotine-tolerant subjects. Cerebral blood flow may also be influenced by changes in respiratory-dependent PCO
2 following nicotinic ganglionic activation. However, such peripheral modulation would likely produce homogeneous alterations in blood flow and blood-oxygen-level-dependent signal rather than the more distributed, heterogeneous pattern of activation we observed. In addition, the blood-oxygen-level-dependent weighted pulse sequence and long interscan time used in this study are heavily weighted to reflect changes in local oxygenation more than blood flow (
36). Changes in regional brain metabolism (oxygen extraction and glucose utilization) are more uniquely linked to neuronal activity than changes in cerebral blood flow, which may potentially reflect more generalized vascular alterations independent of regional brain activity, as in CO
2-induced vasodilation (
37).
Taken together, these data suggest that the observed functional MRI signal changes reflect alterations in neuronal activity secondary to CNS nicotinic receptor activation. The data from this study are the first to describe the CNS regions acted upon by acute nicotine administration in humans, are neuroanatomically consistent with the mood-elevating and anxiolytic effects often ascribed to cigarette smoking (
19), and support our hypothesis that the observed cortical activation underlies many of the behavioral properties of the drug. By revealing the regional activation and, importantly, the time course of nicotine's action in the human brain (a feature that functional MRI is uniquely qualified to examine), these data support the role of nicotine in the behavioral effects observed during cigarette smoking. Notably, the real-time functional MRI signal in this study peaked at approximately 2.8 minutes after drug administration and is consistent with the peak plasma nicotine concentration observed at approximately 2 minutes and the reported peak behavioral effects.
Finally, it should be noted that other nicotine-induced functional MRI waveforms were also occasionally observed but not analyzed for this report. For example, some waveforms increased at an appropriate postinjection time but did not return to baseline during the a priori allotted time window for analysis. Whether this response pattern reflects a neuronally induced, alternative drug effect (e.g., prolonged activation) is currently under investigation. For example, it might be posited that multiple brain timing circuits are engaged by cigarette smoking, leading to behaviors with different time constants, i.e., variable interpuff intervals (in tens of seconds) and intercigarette intervals (tens to hundreds of minutes). Only real-time measurements such as functional MRI have the potential to identify brain regions responsible for distinct portions of this complex behavior. It may now be possible to separate the CNS sites responsible for nicotine's reinforcing properties from the drug's other pharmacological effects. This would permit testing hypotheses about the commonality of mechanisms in abused drugs as well as serve as a powerful “bioassay” in the development of agents to manage and treat nicotine abuse.