Personality is perhaps the most unique aspect of our individuality. It is a pattern of behavior and reactions that varies among individuals but within an individual is stable over time. Personality has traditionally been conceptualized as consisting of several factors or dimensions. Although there continues to be spirited debate among personality theorists concerning the exact number of dimensions (
1–
9), most major current models of personality include the dimension of introversion/extraversion at a fundamental level (
10). Not only do most models incorporate introversion/extraversion, but they also have very similar conceptualizations of the construct. For example, Zuckerman, and colleagues (
11) found that ratings of extraversion generated by the two most frequently used personality tests, the Eysenck Personality Questionnaire (
12) and the NEO Personality Inventory—Revised (
13), correspond closely. At one end of the introversion/extraversion continuum are extraverts, who are described as gregarious, socially active, cheerful, assertive, and easily excitable. At the other end of the spectrum are introverts, who tend to be reclusive and quiet, preferring books to other people.
There have been several attempts to describe biological bases of normal and abnormal personality (
1,
3, 5,
6). Eysenck (
3–
5) proposed that behavioral differences between introverts and extraverts result from an innate drive to compensate for overactive and underactive reticulo-thalamo-cortical pathways, respectively. Gray (
6,
14) conceptualized extraverts as having lower activity than do introverts in the behavioral inhibition system, a functional loop that includes the ascending reticular activating system, the frontal lobes, septal regions, and the hippocampus. According to both of these models, introverts have higher cortical activity than extraverts, especially in the frontal lobes. The models differ in terms of the mechanisms responsible for differences in cortical activity.
Functional imaging allows researchers to examine metabolic or blood flow correlates of specific cognitive processes and mental states. These technologies have the potential to directly measure functional differences that may exist in the brains of extraverts and introverts.
Few studies have used functional imaging techniques to explore the relation between brain activity and personality in healthy individuals. Mathew et al. (
15) examined the relation between extraversion and cerebral blood flow (CBF) in a group of 51 female subjects with the use of xenon inhalation and cortical probes. They found a negative relation between the two variables, with lower overall blood flow in highly extraverted individuals. Stenberg et al. (
16) attempted to replicate these findings in a group of 19 male and 18 female subjects with the use of xenon single photon emission computed tomography. Mean CBF was related to extraversion only when the sample was limited to female subjects of the same age as those in the Mathew et al. study. In addition to whole brain blood flow, Stenberg et al. examined regional blood flow and found lower flow in the temporal lobes in extraverts relative to introverts.
Neither of the studies of extraversion mentioned above used standardized stereotaxic coordinate systems or aligned functional imaging data with anatomical magnetic resonance imaging (MRI) or computerized tomography images. Thus, these studies were not able to accurately describe the exact location or spatial extent of regional blood flow patterns associated with extraversion. The positron emission tomography (PET) technique provides more precise spatial resolution than the methods described above and is able to measure blood flow from the entire brain. PET has been used extensively to describe the circuitry subserving various aspects of normal cognition. To our knowledge, the present study was the first to utilize PET imaging to examine the relation between regional CBF and the personality dimension of introversion/extraversion in normal individuals.
Normal subjects engaging in a series of loosely connected personal recollections and plans for future activities activate large regions of the frontal lobes (
17), while memory retrieval specifically activates the right frontal cortex (
17–
23). To the extent that introverted individuals engage in more cerebral activities (thinking, planning, remembering) than do extraverts, activity in regions associated with these functions, namely the frontal lobes, would be expected to be higher in introverts than in extraverts.
METHOD
The subjects of this study were 18 healthy normal individuals (10 male and eight female) recruited from the community. Their mean age was 28.9 years (SD=8.5, range=19–48), and their mean level of education was 14.2 years (SD=1.7, range=12–18). All subjects were screened for exclusionary criteria including prior psychiatric, neurologic, or serious medical conditions. Before participation all subjects gave written informed consent to the protocols, which were approved by the University of Iowa Human Subjects Institutional Review Board.
Introversion/extraversion scores were taken from the NEO Five-Factor Inventory (
13), a 60-item abbreviated form of the NEO Personality Inventory—Revised. The extraversion scale of the NEO Five-Factor Inventory correlates highly (r=0.90) with the corresponding scale from the NEO Personality Inventory—Revised. It has demonstrated internal consistency (coefficient alpha=0.77) and test-retest reliability (at 3 months, r=0.79, N=208, p<0.001). In the present study percentile scores were used, based on separate gender norms as suggested in the test manual. Scores on the extraversion scale ranged from 31 to 75 (mean=53.8, SD=12.5). Men and women did not differ significantly in terms of extraversion scores.
Regional CBF was measured using the bolus water ([
15O]H
2O) method (
24–
26) with a GE 4096 PLUS scanner. The subjects were oriented in the PET scanner with laser light guides aligned at the orbitomeatal line. The center of the most rostral slice was indicated by the laser guides. Fifteen slices (6.5 mm center to center) with an intrinsic in-plane resolution of 6.5 mm full width at half maximum and a 10-cm axial field of view were acquired. Imaging consisted of 20 5-second frames. To determine the time course of bolus transit, time-activity curves were generated for regions of interest placed over major vessels within the brain. The eight frames representing the first 40 seconds immediately after bolus transit were summed to make a composite image. This composite image was reconstructed into 2-mm pixels in a 128×128 matrix with a Butterworth filter (order=6, cutoff frequency=0.35 Nyquist). With use of the above-described image, blood curve (PET counts/pixel per second), and an assumed brain partition coefficient of 0.90, the regional CBF (expressed in milliliters per minute per 100 grams of tissue) was calculated pixel by pixel on the basis of the equation described for the autoradiographic technique (
25). For a more detailed description of the method, see Hichwa et al. (
27) and Hurtig et al. (
28). During image acquisition, subjects were instructed to lie quietly with their eyes closed. No further instructions were given for this condition.
The MRI images consisted of contiguous coronal slices (1.5 mm thick) acquired on a 1.5-T GE Signa scanner. Technical parameters of the MRI acquisition were as follows: spoiled gradient/recall acquisition sequence, flip angle=40°, TE=5 msec, TR=24 msec, number of excitations=2. MRI images were transferred to the Image Processing Laboratory of the University of Iowa Mental Health Clinical Research Center for analysis with the use of Silicon Graphics workstations and locally developed software (BRAINS) (
29–
34.
The initial step of postacquisition processing involved “removing” the brain from the skull with a combination of automated edge detection techniques and manual tracing. Pixels representing surface CSF were classified through a thresholding procedure and removed from the display. To ensure comparability of head position across subjects, the scans were volume rendered, realigned parallel to the anterior commissure/posterior commissure line and the interhemispheric fissure, and placed into standard Talairach coordinate space (
35). At this point, images from multiple subjects or multiple scans from a single subject can be coregistered. Finally, images were resliced in three orthogonal planes to produce a three-dimensional data set that was used for visualization and analysis.
The quantitative PET blood flow images were transferred to the Image Processing Laboratory for further analysis (
29–
34). The first step in image analysis involved registration of each individual’s PET and MRI images. This coregistration consisted of a two-stage process. Initially, a coarse fit based on surface matching of the MRI and PET images was generated (
36); then, with the surface fit data as input, a variance minimization program (
37) was used for the final coregistration. Brain landmarks identified on the MRI were used to place each coregistered image into standardized coordinate space (
35). An 18-mm Hanning filter was applied to the PET images to eliminate residual anatomical variability.
Mean whole brain blood flow (milliliters per minute per 100 grams of tissue) was computed from PET flow within the voxels contained in the brain.
Locally developed software similar to that used by other groups (
38–
43) was used to calculate correlational analyses of regional blood flow. Resolution was reduced from a voxel size of 1.08 mm × 1.35 mm × 1.48 mm to 3.00 mm × 2.70 mm × 2.00 mm. Voxel data are treated as a collection of data vectors; the length is defined by the number of subjects. A Pearson correlation between blood flow within each voxel and introversion/extraversion scores was calculated; this resulted in a three-dimensional correlation matrix displaying correlation coefficients for every voxel. This correlation image presents an overall pattern of the relation between blood flow and introversion/extraversion. A threshold can be applied so that only regions with a correlation coefficient above a certain value are displayed. Selection of a threshold in imaging research remains difficult because of the sheer number of voxels involved in the correlational analysis and the lack of independence of contiguous voxels. For the present study, a correlation threshold of 0.60 (p<0.005, uncorrected for multiple comparisons) with introversion/extraversion and a size criterion of at least 10 contiguous voxels were selected.
RESULTS
To investigate the relation between introversion/extraversion and whole brain blood flow in this study group, a Pearson correlation coefficient was computed. Contrary to previous findings (
15,
16), introversion/extraversion did not account for a significant amount of the variance in whole brain blood flow (r=–0.21, N=18, p=0.20).
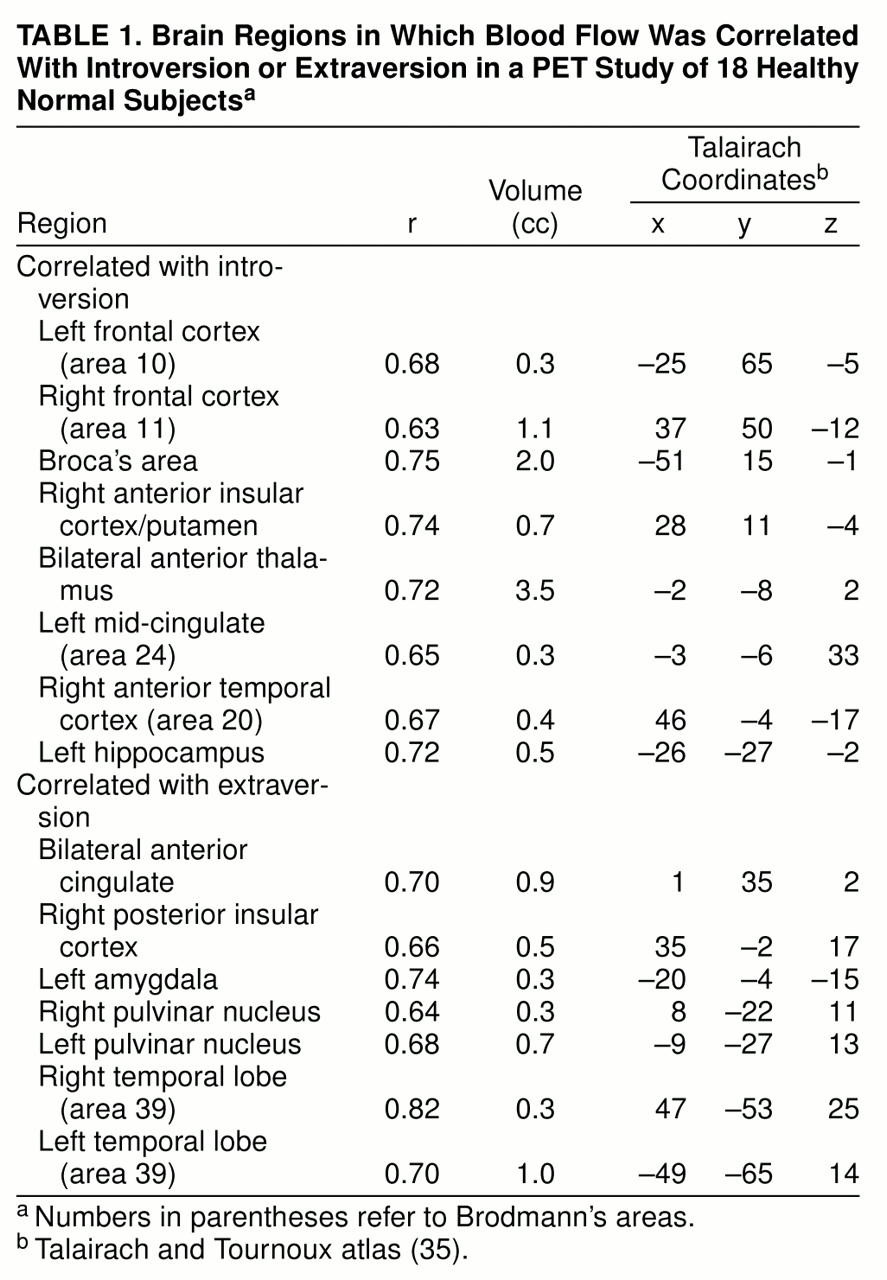
The analysis identified 17 regions that exceeded a correlation of 0.60 with introversion/extraversion and a size of 10 contiguous voxels. The locations of these regions in standardized space as well as the size and correlation coefficient of the center voxel are presented in
table 1. It should be noted that the correlational analysis was performed with the use of the entire continuum of introversion/extraversion scores. For ease of discussion, areas that were positively correlated with scores on the NEO Personality Inventory—Revised will be described as regions associated with extraversion. Similarly, areas negatively correlated with scores on the NEO Personality Inventory—Revised will be described as regions associated with introversion.
There were eight regions correlated with introversion and seven correlated with extraversion. Regions showing a relationship with introversion had a larger volume than did regions associated with extraversion (10.7 cc versus 4 cc).
In line with predictions, introversion was correlated with blood flow in the lateral extent of the frontal cortex, Broca’s area, the insular cortex, the right temporal cortex, and the anterior nucleus of the thalamus. Further, several cortical regions were found to be correlated with extraversion. These regions included the anterior cingulate gyrus, right insular cortex, bilateral temporal lobes, and pulvinar nucleus of the thalamus.
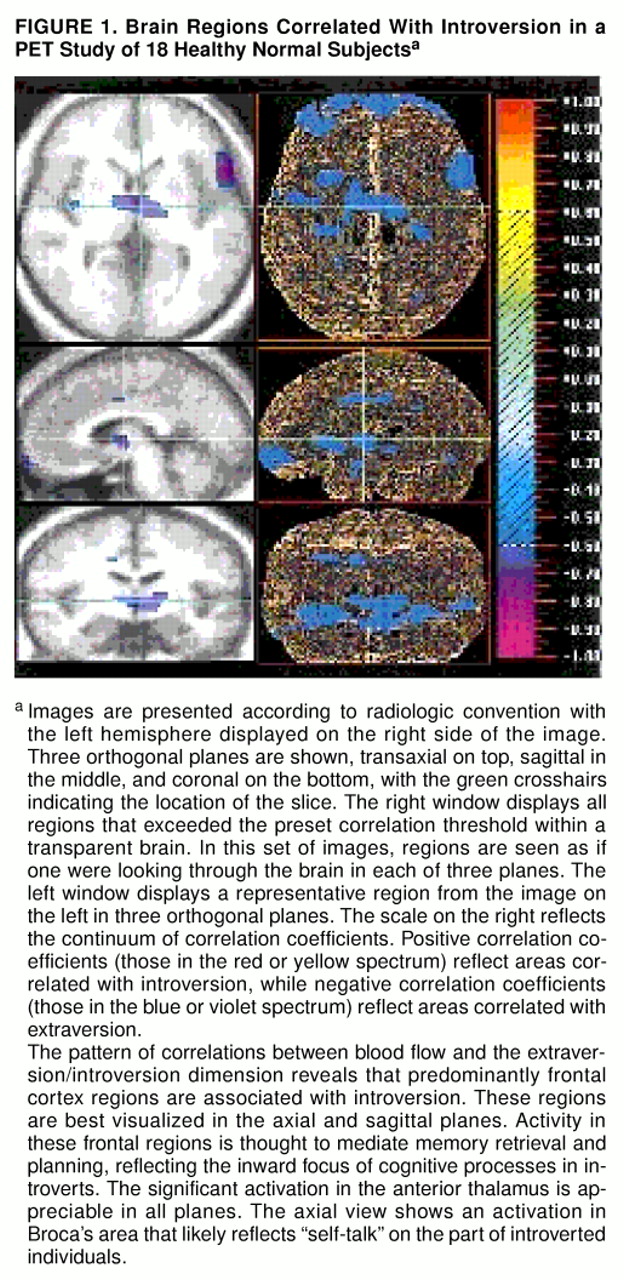
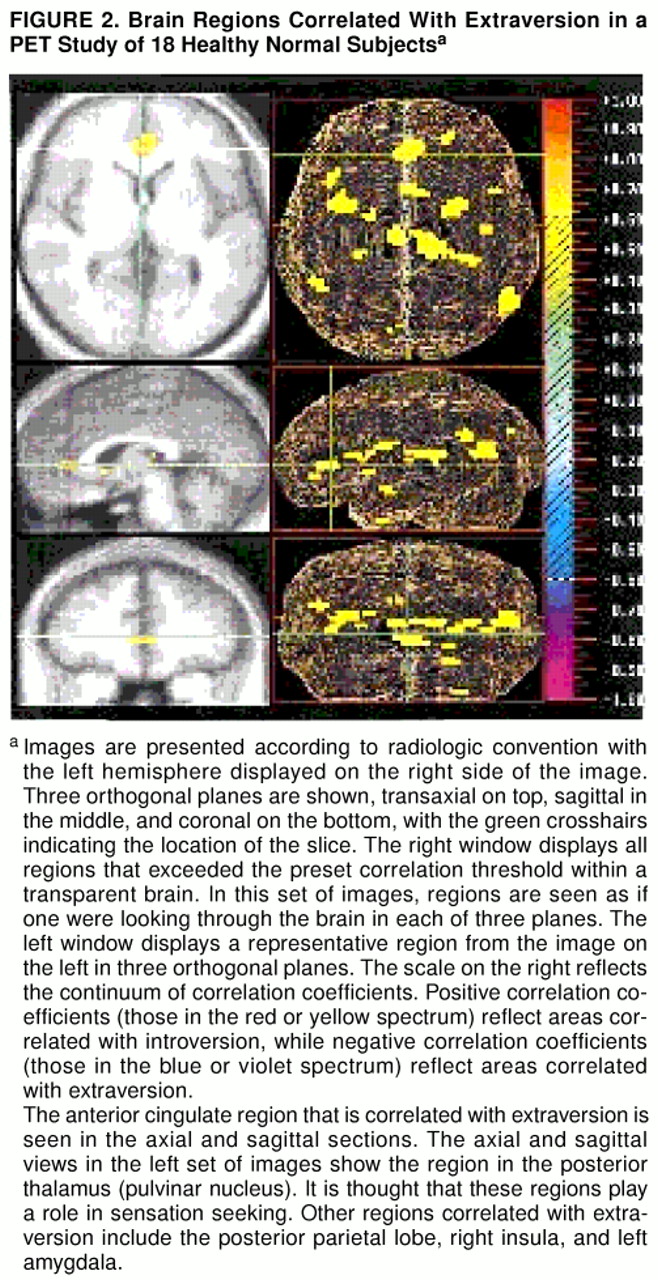
Figure 1 and
figure 2 depict regions correlated with introversion and extraversion, respectively.
DISCUSSION
This study revealed a pattern of increased blood flow in the frontal lobes associated with introversion. This pattern generally supports both Eysenck’s and Gray’s biological theories of personality (
4–
6,
14) and is consistent with results of previous studies (
15,
16,
44,
45). Eysenck’s model (
4,
5) proposes that introverts have higher cortical activity than extraverts; indeed, in the present study, there were more cortical regions associated with introversion than with extraversion. Gray suggested lower than normal activity in the behavioral inhibition system in extraverts; in the present study, extraverts did show lower blood flow in several regions in the behavioral inhibition system, namely, the frontal lobes and the hippocampus.
Blood flow measures were acquired while subjects were free to think about anything, providing a picture of the activity of the undirected and uncensored mind. Normal subjects have reported engaging in a series of loosely connected personal recollections and plans for future activities during the uncontrolled cognitive state (
17). Higher flow in bilateral frontal lobe regions suggests that introverts were engaged in frontally based cognition, including remembering events from their past, making plans for the future, or problem solving. In addition, Gale (
46) speculated that introverts might engage in a running monologue in the absence of external stimulation. The observed increased blood flow in Broca’s area in introverts might be interpreted as biological evidence of “self-talk.”
The present study is the first to describe thalamic regions associated with introversion/extraversion. The thalamus has long been recognized as an obligatory relay station for sensory information headed for the primary sensory cortices. Changes in the activity of the thalamic nuclei have the potential to decrease or increase the amount of information reaching cortical regions. Two regions, a negatively correlated region in the anterior nucleus and a positively correlated region in the pulvinar nucleus, were identified. The anterior thalamus projects primarily to the anterior cingulate gyrus, while the pulvinar nucleus projects mainly to visual cortex and sensory association areas (
47,
48).
This study found that blood flow in the anterior insula is correlated with introversion, whereas blood flow in the posterior insula is correlated with extraversion. Recent studies of emotion have found that the anterior portion of the insula is activated when subjects recall or imagine events, whereas the posterior insula is activated by interpretation of current sensory information (
49,
50). The distinction between an inward focus and an outward focus is very much the difference between introverts and extraverts. Stenberg et al. (
16) reported that extraverts have higher flow in the temporal lobes than do introverts, but because of technological limitations they were not able to localize this region more accurately. It is likely that the increased flow in extraverts was based in the posterior insula.
The greater activity in the anterior thalamic nuclei and frontal lobe regions in introverts likely reflects the introspective nature of these individuals. The posterior thalamus and anterior cingulate regions that were found to be more active in extraverts may underlie these individuals’ high drive for sensory and emotional stimulation.
Although this study used a resting condition, there are many other equally valid conditions for assessing differences in CBF associated with extraversion. Future research should explore these differences in the context of various cognitive tasks, e.g., memory paradigms. Future research could also explore the relation between CBF and other personality dimensions for which biological substrates might be hypothesized (e.g., neuroticism).
While the PET technique allows researchers to explore the relation between activity in brain regions and various cognitive states and brain illnesses, it is not without limitations. Although state-of-the-art analysis techniques are used, the problems of possible edge artifacts, imperfect image registration, and limited spatial resolution remain.
The present study did not provide a definitive answer concerning the relation between personality and brain activity. It does, however, add to a growing literature which suggests that human personality traits are based on individual differences in brain function. This study, combined with others, suggests that frontal cortical regions are highly active in introverts, while more posterior regions are active in extraverts. Moreover, findings of this study suggest that a circuit involving the frontal lobes, the striatum, and the thalamus plays an integral role in modulating individual differences in extraversion.