The pharmacological treatment of schizophrenia is focused on the treatment of positive symptoms. The goal is the maintenance of clinical stability (maintenance therapy), prevention of exacerbations (prophylactic treatment), or the treatment of an episode of positive symptom exacerbation. Conventional and new antipsychotic medications represent the primary pharmacological approach. Other indications have led to an expansion of the pharmacological armamentarium beyond antipsychotic medications. Lithium, antiseizure drugs, and antianxiety drugs have been considered in an effort to enhance antipsychotic drug treatment for the treatment-refractory patient; and other medications have been introduced to address associated problems (such as antidepressant drugs for depression, hypnotics for sleep, and beta blockers for aggression) or to reduce antipsychotic side effects (
1–
6). There is also increasing attention to the pharmacotherapy of negative symptoms (
7), including preliminary efficacy data for glycine and
d-cycloserine (
8–
11).
Despite this range of drug therapy, the prodromal symptoms of impending psychotic exacerbation have not been isolated as a unique target of drug treatment. Rather, early detection of relapse has been viewed as an indication for initiating antipsychotic drug treatment (for patients who are not taking medication); for increasing the dose of medication (for those receiving continuous maintenance medication); for psychosocial interventions to decrease stress, provide emotional support, and ensure close observation; and for careful observation of the developing psychopathology to clarify the diagnosis in the case of a patient’s first episode. However, subtle clinical changes preceding psychotic relapse are common. The availability of a pharmacological treatment other than typical and atypical antipsychotics would enhance dose reduction strategies and provide a drug treatment for patients refusing antipsychotic drugs and might generalize to the prodromal symptoms preceding the first psychotic episode, when the physician understandably hesitates to administer antipsychotic medication before the appearance of frank psychotic symptoms.
The development of treatments for this phase of illness depends on a valid definition of the changes in clinically stable outpatients that indicate impending relapse. Some authors assert that psychotic symptoms cannot be used to define a prodrome to psychosis (
12). Retrospective (
13) and prospective (
14) studies suggest that a number of changes other than psychotic symptoms warn of impending relapse. These include disturbed sleep, increased anxiety or other dysphoric affect, agitation and irritability, increased suspiciousness, and peculiar perceptual experiences. Since many stable patients are in only partial remission, clinical reasoning requires any increase from stable baseline psychotic symptoms to be considered a sign of impending relapse. It is difficult to imagine clinicians’ not responding to these clinical changes as an indication for treatment to prevent relapse, even though relapse may not be inevitable. We have shown that a broad definition that includes changes in both psychotic and nonpsychotic signs and symptoms has high sensitivity and specificity for the prediction of relapse (
15).
Several considerations suggest that antianxiety drugs, especially benzodiazepines, would be effective in the prevention of relapse if treatment were focused on these early warning signs. Mild psychotic symptoms in nonschizophrenic illness often decrease with antianxiety medication. Benzodiazepine drugs diminish neurotransmission in dopaminergic systems through γ-aminobutyric acid feedback mechanisms (
16), and all antipsychotic medications, both conventional and new, are thought to work in part through reduced dopamine neurotransmission. There is also evidence that supplementing antipsychotic treatment with a benzodiazepine during periods of florid psychosis reduces agitation (
17). However, efforts to substitute benzodiazepines for antipsychotic drugs in treating full psychotic episodes have not proven effective because high doses were required, producing unacceptable levels of sedation (
17). In addition, lower-dose benzodiazepines, as adjuncts to antipsychotic medication, have not proven very effective at enhancing therapeutic outcome in partially responsive patients (
17,
18).
In a preliminary open-label study (
19), we reported that diazepam, in doses ranging from 10 to 40 mg/day, appeared to be effective in preventing relapse in nine drug-free patients with schizophrenia who presented with either psychotic or nonpsychotic early warning signs of relapse. These results support the potential utility of benzodiazepines in preventing progression of a psychotic exacerbation, and we therefore made the one-sided hypothesis that diazepam (compared to placebo) would be efficacious in preventing progression of a psychotic exacerbation. To test this hypothesis, we conducted a double-blind, randomized clinical trial comparing the efficacy of diazepam, fluphenazine, and placebo for treating early warning signs of relapse. Fluphenazine was included in the design to determine whether the study cohort was treatment responsive (in the case that placebo and diazepam were equally effective) and to compare diazepam to standard antipsychotic therapy if diazepam proved superior to placebo. The last two experimental conditions were therefore exploratory and not hypothesis driven.
METHOD
Subjects
Fifty-three patients who met either DSM-III-R criteria or Research Diagnostic Criteria for schizophrenia or schizoaffective disorder were selected from the Maryland Psychiatric Research Center Outpatient Research Program for entry into the study. Patients were diagnosed by using a best-estimate diagnostic approach that used all available information from a structured diagnostic interview (Structured Clinical Interview for DSM-III-R), direct assessment, family informants, and past medical records. Patients with concurrent drug abuse or alcoholism, organic brain disorders, mental retardation, or any history suggesting that medication withdrawal would pose an undue risk to the health or safety of the patient or others were excluded from the study. Following an informed consent process, which considered risks and alternative treatment approaches, all patients provided written informed consent. The consent procedures were approved by the university’s institutional review board.
Study Design
All antipsychotic medication was withdrawn, without taper and on an open basis, from clinically stable patients. During the protocol period, all aspects of psychosocial treatment were continued, and close clinical monitoring was provided to ensure safety and early detection of any exacerbation. After withdrawal of daily antipsychotic therapy, pharmacotherapy was defined by early detection and rapid intervention, with drug selection determined by protocol design. Patients were seen at least weekly by a master’s- or doctoral-level clinician. The dose and type of medication before withdrawal were based on clinician’s choice and were not standardized across the population; all patients were receiving conventional antipsychotics. If the patient was observed to exhibit a change in clinical status, which could potentially represent early warning signs of exacerbation, then Brief Psychiatric Rating Scale (BPRS), Clinical Global Impression (CGI), and sleep change ratings were obtained. If a patient met threshold criteria for early warning signs (discussed later), then he or she was given oral diazepam, fluphenazine, or placebo in an all-blind design. A 7-day per week, 24-hour per day on-call service, provided by clinic staff, was available to patients and their families in order to evaluate patients immediately if symptoms occurred during nonclinic hours. The same service was available to patients after their random assignment to one of the three pharmacological treatments.
A stratified randomization procedure across the 53 patients was used to assign drug treatment to balance study groups on gender, prior social function, and past duration of hospital care (
20). The diazepam group had 15 patients, the fluphenazine group had 18, and 20 patients received placebo. The daily dose of diazepam was 10 mg t.i.d., and the dose of fluphenazine was 5 mg t.i.d. Patients were treated at these doses for 4 weeks. In week 5 patients received two tablets per day, and in week 6 they received one tablet per day; this 2-week period of drug taper was single-blind. The taper period was designed to minimize the risk of diazepam withdrawal reactions. During the double-blind phase, decreases in dose and the use of antiparkinsonian agents were prescribed for the treatment of side effects.
BPRS and CGI ratings were obtained during each of the weekly visits and were used to assess the patients’ baseline and change in symptoms (discussed later). The interrater reliabilities were 0.80 and 0.79, respectively, for BPRS total score and the CGI global item. If patients complained of or exhibited further worsening of symptoms and met a priori criteria for exacerbation of symptoms beyond that which initiated double-blind treatment, they were removed from the study and treated on an open basis with fluphenazine. This procedure is based on targeted drug treatment principles (
21), which have produced results similar to standard care in this clinic (
22) and exhibit relative safety as a 2-year treatment (
22,
23).
Clinical Assessments
The following criteria were used to define early warning signs of relapse (both A and B were required):
A. The patient was considered by the therapist and physician pharmacotherapist to be significantly more symptomatic than at baseline, and an intervention was indicated.
B. One or more of the following:
1. Relative to the baseline drug-free BPRS rating, there was an increase of 2 or more points on one or more of the following BPRS items: somatic concern, anxiety, conceptual disorganization, hostility, suspiciousness, or hallucinatory behavior;
2. The patient had a new complaint of marked insomnia, the family reported a change in sleep pattern, or both;
3. There was an increase of 2 or more points on the CGI or an increase from 6 to 7 (note that average score at entry was 3.5 [between mild and moderate]).
The A criterion was designed to minimize the influence of normal fluctuations in symptom severity on the judgment of early warning signs of relapse. The B criterion was based on previous studies of the most commonly observed early warning signs (
13,
14).
The following criteria were used to define further exacerbation and therefore a failure of the initiated drug treatment (both A and B were required):
A. The patient was considered by the therapist and physician pharmacotherapist to be significantly more symptomatic than at the time early warning signs of relapse were identified, and an intervention was indicated.
B. One or more of the following:
1. Relative to the BPRS rating when early warning signs of relapse were identified, there was an increase of 2 or more points, or a change from 6 to 7, on one or more of the following BPRS items: somatic concern, anxiety, conceptual disorganization, hostility, suspiciousness, hallucinatory behavior, or unusual thought content;
2. An increase of 2 or more points on the CGI or an increase from 6 to 7.
Statistical Analyses
Survival analysis was used to assess the primary outcome variable, occurrences of further exacerbation following drug treatment. The primary hypothesis was the superiority of diazepam compared to placebo. Baseline antipsychotic dose was assessed by using the conversion system developed by Schooler and colleagues (
24).
RESULTS
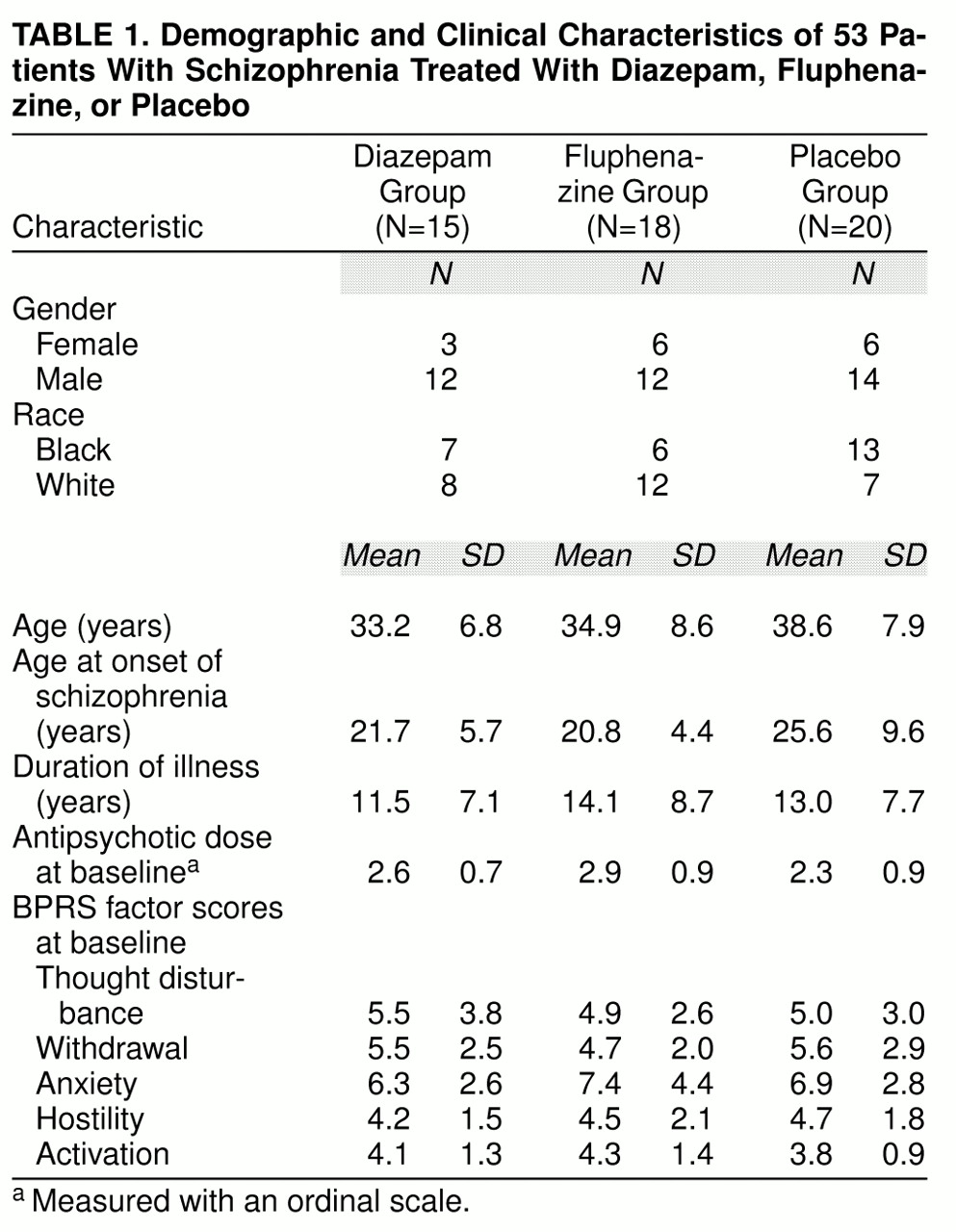
The treatment groups were similar with respect to demographic features and baseline antipsychotic dose (
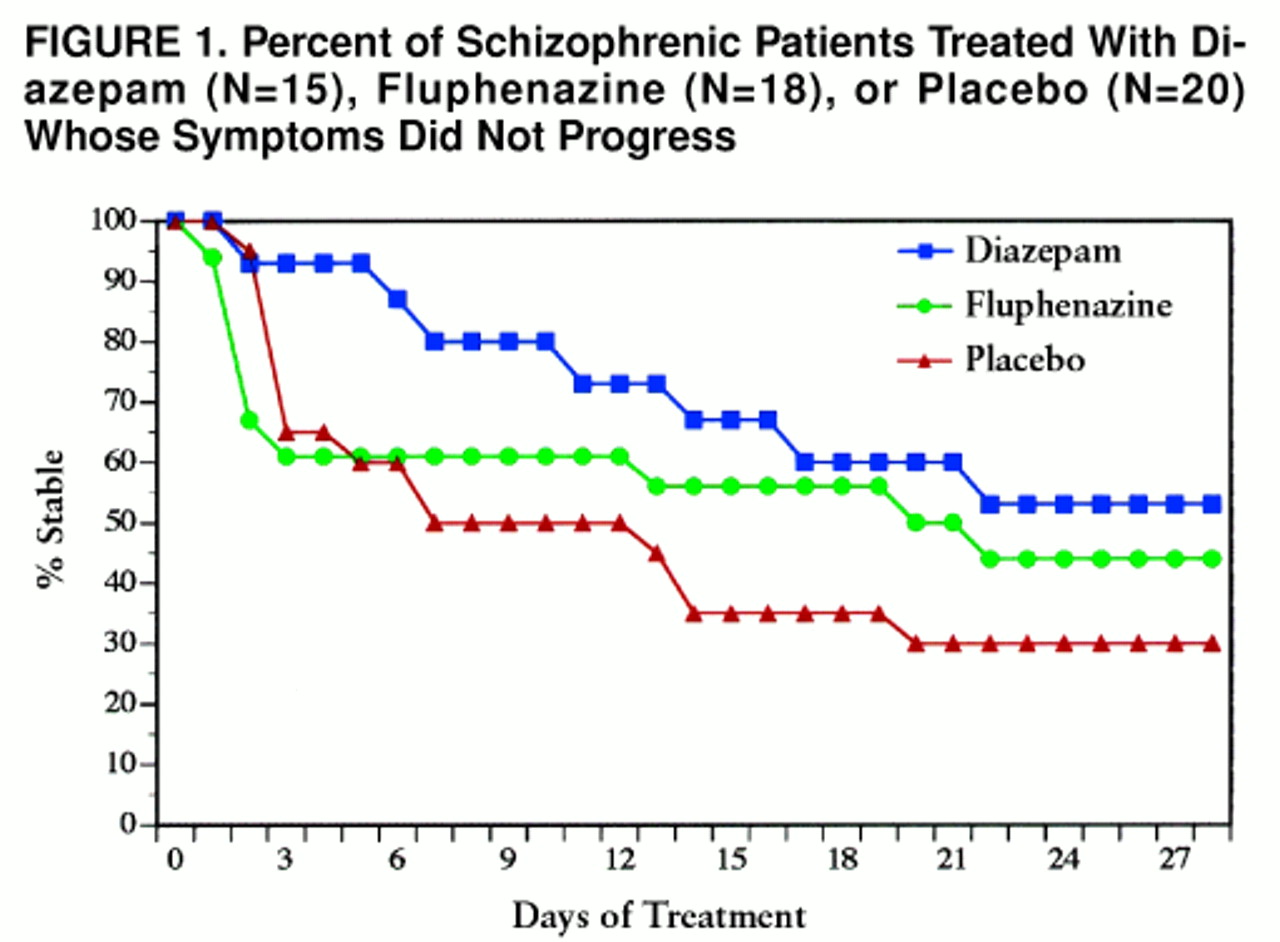
table 1). The primary hypothesis, the superiority of diazepam compared to placebo, was tested with the Peto-Wilcoxon statistic (z=1.81, p=0.04, N=35) (
figure 1). At the end of the 4-week treatment period, 30% (N=6) of the placebo-treated patients and 53% (N=8) of the diazepam-treated subjects had not advanced to the next stage of exacerbation.
The inclusion of the fluphenazine arm was designed to examine whether the study cohort was treatment responsive, in the event that placebo and diazepam were equally effective, or to compare diazepam with standard antipsychotic therapy if the former proved superior to placebo. Only the latter comparison is germane, since it was demonstrated that diazepam was superior to placebo. There was no statistically significant difference between fluphenazine and diazepam treatment according to the Peto-Wilcoxon statistic (z=1.06, p=0.29, N=33) (
figure 1). Forty-four percent (N=8) of the fluphenazine-treated subjects and 53% (N=8) of the diazepam-treated subjects had not advanced to the next stage of the relapse by the end of treatment.
DISCUSSION
Continuous administration of antipsychotic medication is the standard treatment recommendation for most cases of schizophrenia, for both relapse prevention and treatment of psychosis (
25). However, the actual treatment experience of patients is far more varied. Poor compliance is more the rule than the exception with conventional antipsychotics (
26,
27), and discontinuation of medication is recommended in many clinical circumstances (e.g., following stability after a first psychotic episode, during pregnancy, on the first appearance of dyskinetic movements, or as a consequence of dystonia or other intolerable side effects). Even in the minority of cases in which dosage is in the recommended range (
28), symptom exacerbation and relapse are not uncommon. The many problems associated with traditional continuous antipsychotic drug treatment will be reduced with new generation drugs, but the extent of this advantage is not yet determined, and a new profile of adverse effects (e.g., weight gain) will continue to complicate clinical management. It is, therefore, desirable to increase efficacious and effective treatment options, including drug therapies with modes of action different from the antipsychotic compounds.
In this double-blind study of the treatment of early warning signs of relapse in outpatients with schizophrenia, we found diazepam to be superior to placebo and similar to fluphenazine in preventing symptom progression. The dose range we found to be effective in this context is relatively low, and withdrawal problems were not encountered within the time and dose parameters of this study.
Considering the current public attention to clinical research that involves medication-free periods for schizophrenic patients (
29), a brief note emphasizing the purpose, safety, and treatment strategy of this study is warranted. We have addressed the general ethical and safety issues elsewhere (
30) and suggest that a considerable body of data supports the proposition that medication-free research has not been associated with any long-term disease disadvantage. In this study, an antipsychotic drug intervention was available to patients if exacerbation progressed despite experimental intervention. This backup antipsychotic drug intervention was implemented at a level of exacerbation associated with successful intervention in the targeted drug studies. These studies used frequent clinical monitoring techniques similar to those employed in the current study. The threshold for the current experimental intervention was at an even earlier stage. The overall expected safety was within the range defined by the targeted drug studies (
31), including the two studies at the Maryland Psychiatric Research Center (
23,
32). The long-term course of patients treated with the targeted approach was similar to that of patients receiving traditional maintenance treatment approaches, while increased problems with symptom exacerbations were usually managed successfully on an outpatient basis. This risk was weighed against the importance of new treatment development to determine whether the experiment met ethical standards, but each patient had to judge for himself or herself whether participation was acceptable. Written informed consent following an informed consent process for patients judged not to be decisionally incapacitated was obtained in each case. The purpose of the study was new treatment development, not to cause and observe relapse. Treatment was active and available at all times during the study, but pharmacologic intervention was protocol based and substituted a targeted antipsychotic treatment for continuous drug treatment.
Experience during the study was generally reassuring. When early signs of exacerbation and relapse were observed, treatment in the experimental phase or the antipsychotic intervention phase was successful for most of the 53 patients. Three patients were hospitalized during the experimental period and three during the subsequent month. Average stay was 17 days, with return to baseline symptom status and a return to the clinic in all cases. Two other patients left the clinic but continued to be followed through regular telephone contact, and both later returned. Neither of these cases involved relapse or rehospitalization, but improvement during the off-medication phase may have played a role in wishing to “go it alone” in one case.
With on-medication relapse rates in the range of 40%–50% per year in standard care and 20%–30% in optimized researched-based care (
26,
27), the observed adverse events may be in the expected range for chronically ill patients with a relapsing form of schizophrenia. Nothing definitive can be stated in the absence of a control group receiving continuous medication. However, an expected rate can be estimated. The six rehospitalizations occurred during a 3-month period before, during, and after experimental treatment protocol. Weiden and Olfson (
27) estimated that during long-term maintenance treatment, about 3.5% of patients will relapse per month in optimal care, with higher rates in what they refer to as “real world” care. On the basis of the optimal care rate, about six serious relapses would be expected in this 53-patient cohort in a 3-month period. This provides a perspective in considering whether the six observed rehospitalizations relate to protocol risks or to the natural course of treated schizophrenia. In these six hospital and two dropout cases, no pattern emerged in relation to placebo, diazepam, or fluphenazine at initial intervention. There were no suicide attempts, violent acts, job loss, or change in living arrangements in the course of this study.
The most substantial limitation of our study is the small number of patients, with limited power to detect differences in clinical efficacy among the drugs. The effect size of the placebo/diazepam difference was 0.47 (a medium effect size), compared to the small effect size of 0.21 for the fluphenazine/placebo difference. Our study provided adequate power to detect a difference between diazepam and placebo, but we did not have adequate power to detect any difference between diazepam and fluphenazine. Other limitations do not negate the validity of our finding but may restrict the generalizability of the results. These factors include patient selection from a research clinic, close clinical monitoring that may not be available in many settings, high compliance rates with the therapeutic intervention, and a staff experienced in detecting early warning signs.
If these results do generalize to other cohorts, then a new strategy for dealing with minor exacerbations and early warning signs of relapse is available. This approach may be particularly helpful for patients who resist resuming antipsychotic medication or for whom antipsychotic dose reduction is the goal. It will be important to determine whether patients taking low-dose continuous antipsychotic medication respond to benzodiazepines at the occurrence of early warning signs. If so, this method may further enhance this dose reduction strategy. However, this therapeutic approach requires further study before its role in the treatment of schizophrenia can be adequately evaluated. In the meantime, clinicians may encounter circumstances in which rapid intervention for impending relapse may be required with patients for whom antipsychotic intervention is not feasible. Diazepam seems a reasonable consideration in light of the strong rationale for its therapeutic effect and in the context of these preliminary data from a double-blind, controlled trial. As in this clinical trial, an antipsychotic medication can be added at the first indication that the diazepam intervention is not preventing symptom progression. For the patient and treating doctor, the choice between drug types is not mutually exclusive but, rather, a question of phase and timing.