Schizophrenia is commonly treated with antipsychotic medication that acts through dopamine receptor blockade. Available medications are not always fully effective, nor are they equally effective across the full range of psychopathology associated with this disease
(1). In addition, they produce significant extrapyramidal symptoms. New antipsychotics
(2,
3) may be more effective in treating negative symptoms and may produce fewer extrapyramidal symptoms. The occurrence of extrapyramidal symptoms appears to be related to dopamine D
2 occupancy in the striatum, and all currently available antipsychotics share an antagonism at the dopamine D
2 receptor.
Research for innovative compounds has been directed in part toward focusing the activity spectrum to specific dopamine receptors. Among the five known dopamine receptors, the D
4 receptor in the D
2 family is of particular interest
(4). It is localized at high density in the frontal cortex, mid-brain, and medulla. A selective D
4 antagonist would not be expected to produce any extrapyramidal symptoms because it is not found in the striatum. In addition, because clozapine has a tenfold greater affinity for the D
4 than for the D
2 receptor
(5), the D
4 receptors could represent the principal target for the superior efficacy of clozapine.
RATIONALE
Fananserin (RP62203) (2-[3-[4-(
p-Fluorophenyl)-1-piperazinyl]propyl]-2
H-naphth[1,8-
cd] isothiazole 1,1-dioxide) is a naphtosultam derivative that possesses a potent 5-HT
2A antagonist profile (K
i=0.37 nM) together with a very selective antidopaminergic activity. Heuillet et al.
(7) found that fananserin antagonized the D
4 receptor subtype (K
i=2.93 nM) but had no affinity for D
1 (K
i>1000 nM), D
2 (K
i=726 nM), or D
3 (K
i>100 nM) receptors. This selectivity (approximately 250 times) was not observed for clozapine (K
i=106 nM for the D
4 receptor versus K
i=206 nM for the D
2 receptor), haloperidol (K
i=2.17 nM for the D
4 receptor versus K
i=1.85 nM for the D
2 receptor), olanzapine (K
i=20.3 nM for the D
4 receptor versus K
i=33.0 nM for the D
2 receptor), or risperidone (K
i=6.80 nM for the D
4 receptor versus K
i=3.37 nM for the D
2 receptor)
(7). Among other receptor types, fananserin also presented a marked antagonism at the α
1-adrenergic receptor (inhibitory concentration=4.3 nM) and some antagonism at the muscarinic M
1 receptor (inhibitory concentration=50 nM).
Experimental pharmacology data
(8) demonstrated that fananserin was active in animal models thought to involve forebrain dopamine systems and/or to reflect antipsychotic activity. Fananserin blocked apomorphine-induced climbing (median effective oral dose=48 mg/kg in mice), blocked amphetamine-induced cognitive perseveration (0.5 mg/kg administered subcutaneously in monkeys), and antagonized the psychomotor effects of phencyclidine (2.5 mg/kg administered orally in rats). However, no effect of up to 40 mg/kg given orally was evidenced on the conditioned avoidance test in rats (unpublished data).
In the head-twitch model of central 5-HT
2A activity, fananserin was potently active and had a long duration of action (median effective oral dose=1 mg/kg in rats)
(8). Other CNS effects observed include potent activity in the elevated plus-maze test predictive of anxiolytic activity (minimal effective oral dose=0.25 mg/kg in mice). Fananserin also showed antiaggressive properties and induced slow-wave sleep. However, the compound appeared to be devoid of myorelaxant or sedative activity
(9).
The human tolerability of fananserin was studied in eight phase I studies: seven studies in 183 healthy volunteers and one study in 26 patients with schizophrenia. The drug was shown to be safe and generally well tolerated. The main adverse event was orthostatic hypotension, which occurred at 300 mg b.i.d. after approximately 8 days of titration in patients with schizophrenia.
The present study was designed to assess the safety and effects of fananserin on clinical symptoms of schizophrenia
(10,
11).
METHOD
Patients
Patients were men and women 18 to 60 years old with a primary diagnosis of schizophrenia according to DSM-IV criteria who were treated at multiple sites of the Fananserin Study Group (appendix 1). At baseline, patients were excluded unless they had a total score of 70 or higher on the Positive and Negative Syndrome Scale
(12) and a rating of at least moderately ill on the Clinical Global Impression (CGI)
(13). Also excluded from the study were patients who had been hospitalized for more than 3 months before random assignment to drug or placebo. A recent history or evidence of current substance abuse as well as confounding medical or neurological problems were also exclusion criteria.
No treatment with antipsychotics, antidepressants, or mood stabilizers was permitted during the entire study period (including screening and washout). Treatment with anxiolytics, hypnotics, antiparkinsonians, or analgesics was restricted to lorazepam, chloral hydrate, benztropine, and acetaminophen, respectively, when required. Institutional review boards at each site gave approval before initiation of the study. Written informed consent was obtained from all patients after they had received a complete description of the trial.
Study Design
Ninety-seven patients were randomly assigned in a double-blind fashion to active treatment (fananserin) or placebo (2:1 ratio). The study was organized into three periods: a screening period including the placebo washout (3–8 days), the double-blind period (4 weeks), and a 2-day placebo washout. Hospitalization was required throughout the entire study. Before administration of the study drug, medical and psychiatric histories were obtained and evaluations were performed, including a 12-lead EKG, urine screens for drugs of abuse, measurement of vital signs, plasma pregnancy tests, and routine clinical laboratory tests. Whenever an abnormality described as unacceptable by the protocol was discovered, the patient was declared ineligible.
Patients started double-blind treatment with one capsule (50 mg of fananserin or placebo) twice a day on day 1. On day 2, the dose was increased to two capsules twice a day and subsequently increased every 2 days by adding one more capsule per intake until a safety concern arose or until the assigned dose of five capsules twice a day was achieved. The final dose ranged flexibly from three to five capsules twice a day up to day 15; the final dose on day 15 was then maintained for the reminder of the study. All but one placebo and one fananserin patient achieved the maximum dose of five capsules twice a day. If any patient was given any forbidden medication during the study (i.e., an antipsychotic), the patient was immediately withdrawn from the study.
The Positive and Negative Syndrome Scale
(12), from which 18 items on the Brief Psychiatric Rating Scale (BPRS) were derived, and the CGI
(13) were used to perform psychiatric evaluations. The modified Simpson-Angus Rating Scale
(14; modified version provided by Dr. Simpson) was used to assess motor symptoms. The ratings were carried out by the same rater for each patient during the trial whenever possible unless a change in rater was documented after a joint evaluation.
All evaluations were performed at baseline, the day preceding the first double-blind medication administration. The Positive and Negative Syndrome Scale was given again on day 4 and was repeated weekly. The CGI and Simpson-Angus Rating Scale were given every 2 weeks. The patients who completed the study were asked to rate their personal evaluation of the treatment after their final visit for medication.
EKGs, laboratory evaluations, and pharmacokinetics of fananserin were measured at baseline, on day 4, and weekly thereafter. All EKGs were measured 2 hours after dose administration to assess the maximal effect of the peak plasma level of the drug; the EKGs were reviewed by an independent cardiologist. Blood samplings for laboratory measurements and pharmacokinetics were drawn before dose administration under fasting conditions. Fananserin plasma levels were measured by using validated gas chromatography/mass spectrometry, achieving a limit of quantification of 2.5 ng/ml for 0.1 ml analyzed. Vital signs were measured daily in both supine and standing positions before dose administration and 2 hours after the morning dose administration during the first 2 weeks and weekly thereafter. All adverse events and their full description were recorded on the case report form and were coded according to the COSTART (Coding Symbols for Thesaurus of Adverse Reaction Terms) dictionary.
Statistical Methods
To ensure a power close to 80% in detecting a difference of 15 points in the mean change in total Positive and Negative Syndrome Scale between treatment groups, and with the assumption of a standard deviation of 25 points
(15) with a two-sided Student’s t test (significance level of 0.05), a minimum of 96 patients was required. Random assignment to active drug and placebo groups was performed in blocks of six patients with an unbalanced ratio of two active drug to one placebo.
The primary measure used for assessment of drug efficacy was the change between baseline and endpoint total scores on the Positive and Negative Syndrome Scale. In the case of premature discontinuation, the score at the last observation available was used for the analysis (last observation carried forward method). The last available visit served as endpoint.
Secondary efficacy measures were Positive and Negative Syndrome Scale subscores (negative symptom, positive symptom, and general psychopathology subscores), BPRS score (derived from the Positive and Negative Syndrome Scale), and CGI severity, improvement, and efficacy scores. Responders were defined as patients who completed the study and showed clinical improvement, defined as a reduction of at least 20% in their Positive and Negative Syndrome Scale total score from baseline to day 29.
Changes in the Simpson-Angus Rating Scale total scores from baseline to endpoint (last evaluation during treatment) were analyzed to evaluate extrapyramidal symptoms.
All analyses were performed on the intent-to-treat population, defined as the patients randomly assigned to active drug or placebo who took at least one capsule of the test drug. Additional factors were analyzed for the patients who stayed in the study until day 29.
In the computation of total scores (efficacy or extrapyramidal symptoms), the total score was considered missing if any of the individual scores were missing. SAS procedures were used to perform all statistical analyses
(16).
At baseline, the two groups were compared for demographic and key clinical characteristics. The number of patients having completed each visit, number and reasons for premature discontinuation, and number and reasons for protocol violations during the course of the study were specified for each treatment group.
The groups were compared for rate of intake of an antipsychotic or any other forbidden treatment. Exposure to the active drug was investigated and confirmed by measuring plasma levels of fananserin (trough values).
Because of the small number of patients recruited at some of the participating sites, the effect of the treating facility was analyzed by combining them into three subgroups: 1) the one facility that recruited a sufficient number of patients (N=33), 2) a pool of the academic-research facilities (seven sites, N=39), and 3) the remaining private clinics (four sites, N=25).
For continuous parametric variables, analysis of variance (ANOVA) was used on the change from baseline to endpoint, with treatment and center as factors (two-way ANOVA). The treatment-by-facility interaction term was then excluded from the model because no significant interaction was found (p>0.10). For binary variables like the percent of responders, the Cochran-Mantel-Haenszel test stratified by facility was used to compare the two treatment groups. For ordinal categorical variables such as CGI and extrapyramidal symptom measures, the nonparametric Wilcoxon test was applied to evaluate the treatment effect.
RESULTS
Patient Characteristics
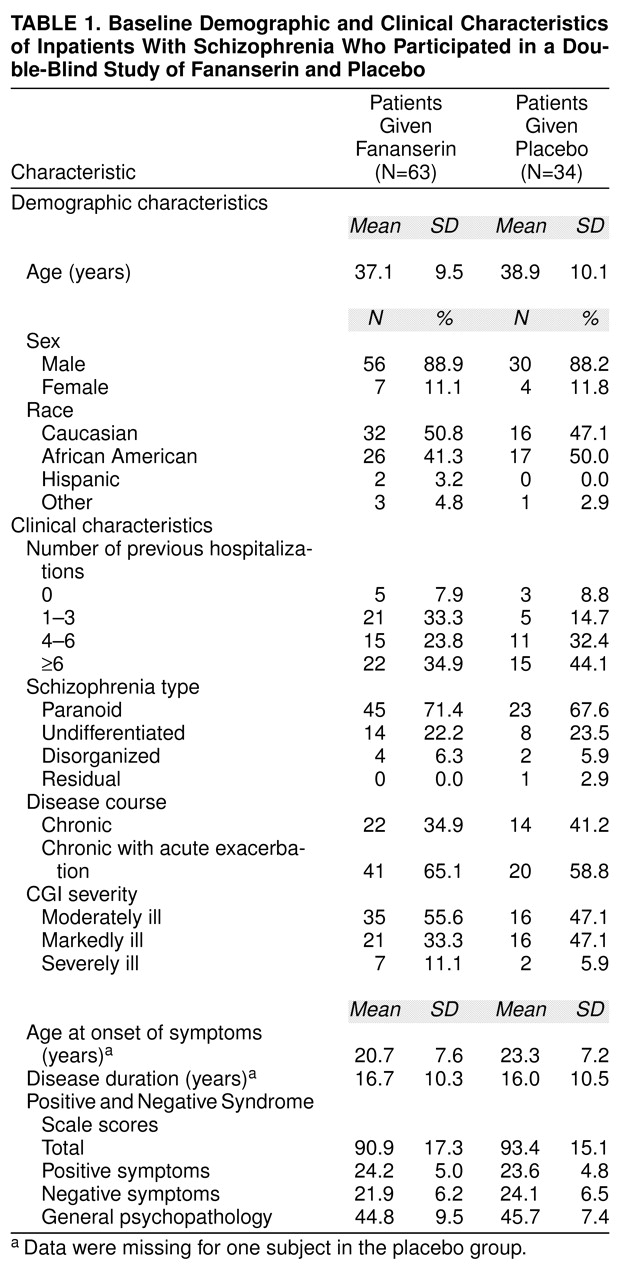
The study was conducted in 12 psychiatric inpatient facilities in the United States. Ninety-seven patients were randomly assigned to active drug or placebo between June 1996 and February 1997. All 97 patients were used in the principal analysis of the intent-to-treat population. Sixty-three patients were assigned to fananserin, and 34 were assigned to placebo. Sixty-nine patients completed the 4 weeks of treatment.
There were no significant differences between treatment groups in demographic or clinical characteristics (
table 1). Eighty-six patients were men and 11 were women; their mean age was 37.7 years (SD=9.7). Patients had been suffering from schizophrenia for a mean of 16 years. The predominant schizophrenic diagnosis was paranoid schizophrenia (68 patients).
Efficacy
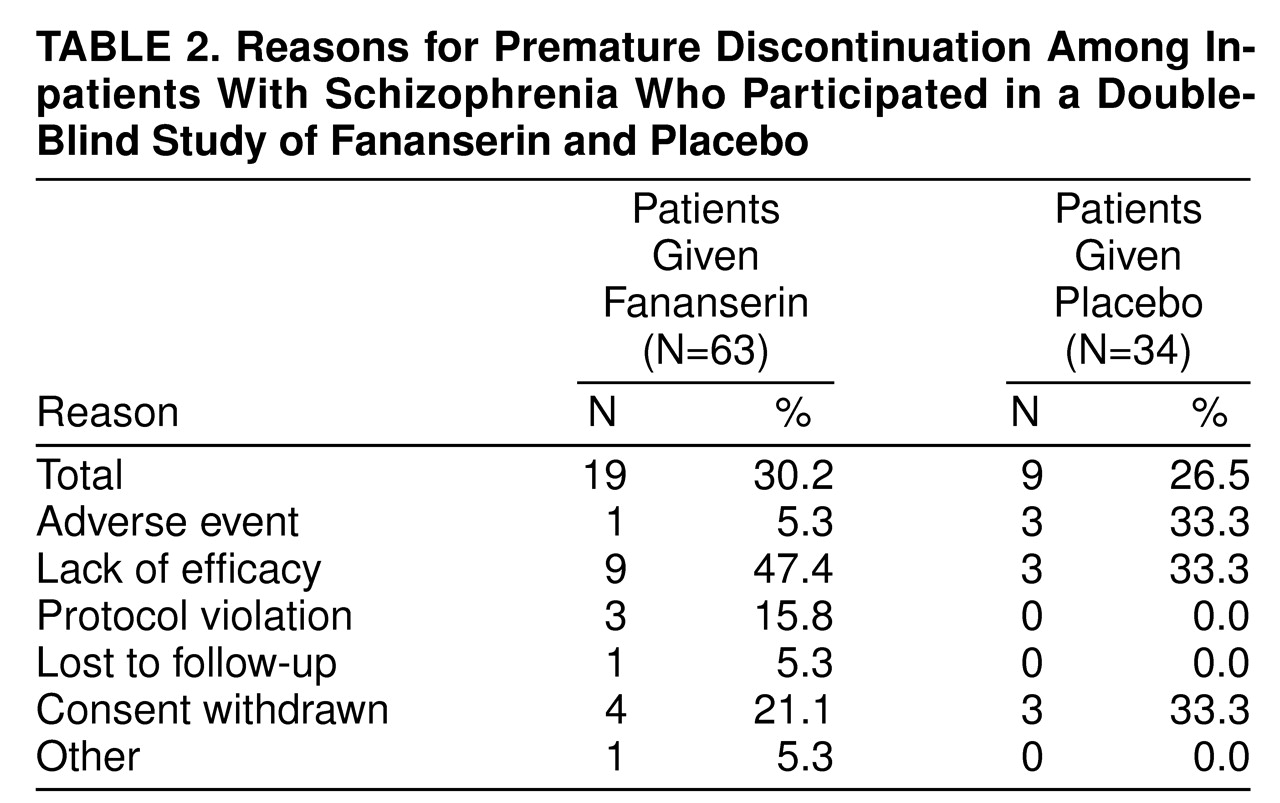
The overall premature discontinuation rates were not different between the two treatment groups (
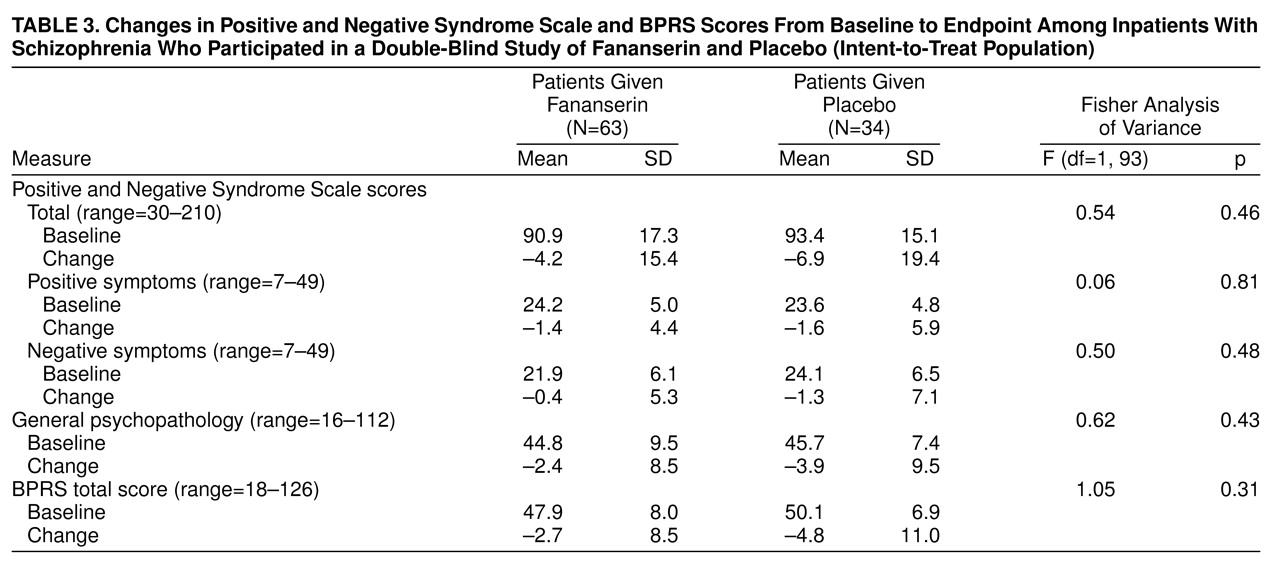
table 2). Similar decreases in total Positive and Negative Syndrome Scale scores were seen in both groups at the endpoint analysis of the intent-to-treat population (
table 3). A lack of difference between the two groups was also found in the Positive and Negative Syndrome Scale subscores for positive symptoms, negative symptoms, or general psychopathology (
table 3) and in the CGI ratings of both severity and improvement. The intent-to-treat analysis showed no effect for facility or any interaction between treatment and facility among the three subgroups of facilities.
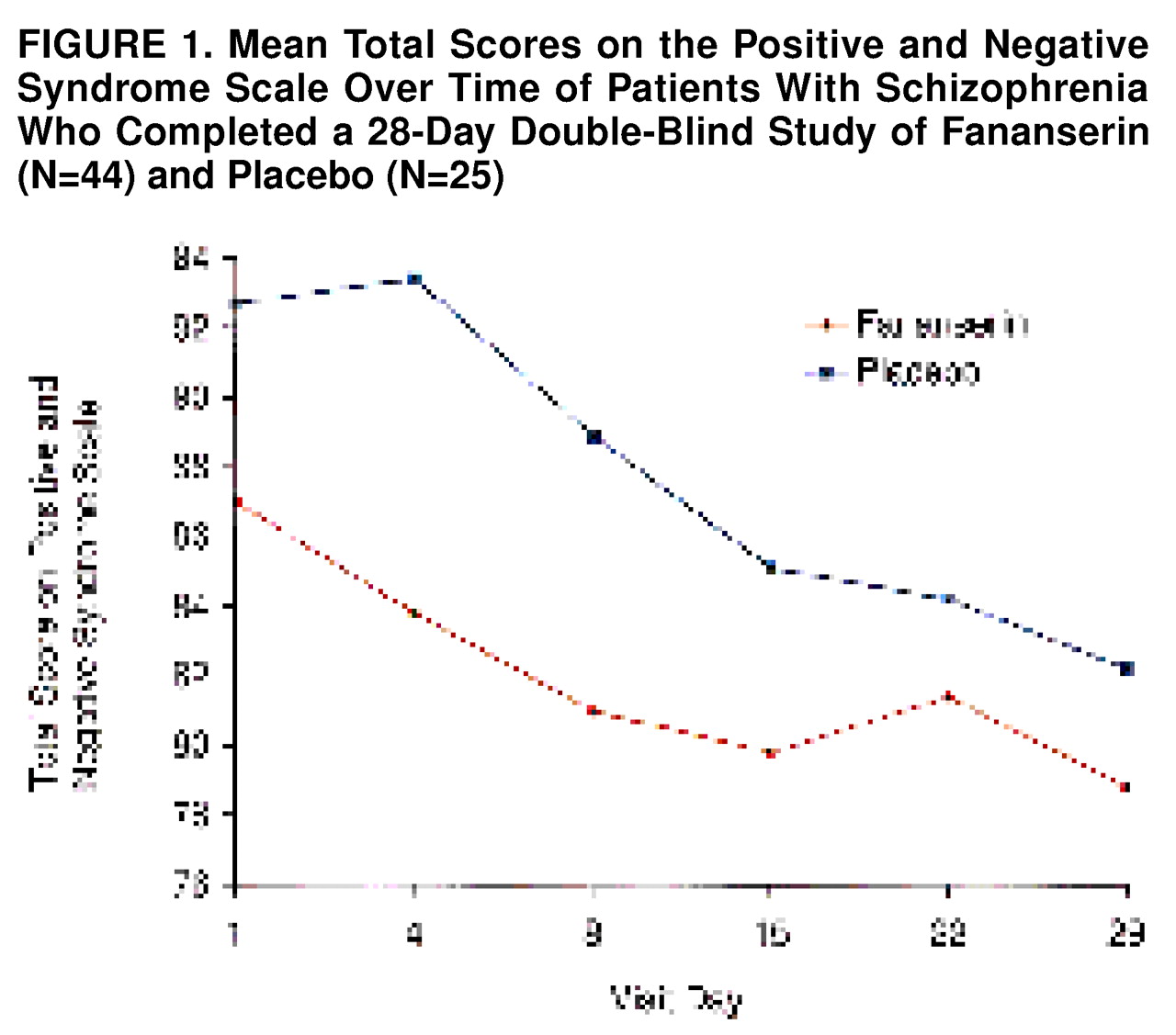
The Positive and Negative Syndrome Scale total scores at each visit among the patients who completed the study are shown in
figure 1.. There was no apparent difference in the evolution of the symptoms between the two treatment groups.
The percents of patients who achieved a reduction of at least 20% in their total Positive and Negative Syndrome Scale score were comparable in both groups: 11 (17.5%) of those given fananserin and eight (23.5%) of those given placebo (Cochran-Mantel-Haenszel χ2=0.51, df=1, p=0.47).
Some patients had a marked improvement at endpoint according to their CGI ratings: one (1.6%) of the patients given fananserin and two (5.9%) of those given placebo were very much improved, and nine (14.3%) given fananserin and seven (20.6%) given placebo were much improved. These patients did not show any specific characteristics of the disease; nor did any Positive and Negative Syndrome Scale item predict this better outcome.
Among the patients who completed the 4-week study, most responded positively to the question, “How did you like the drug?”: 31 (70.5%) of the 44 patients given fananserin and 19 (76.0%) of the 25 patients given placebo liked it from a little to very much.
Extrapyramidal Symptoms
Patients had almost no extrapyramidal symptoms at study entry (i.e., after withdrawal of their current antipsychotic medication). The baseline levels of extrapyramidal symptoms according to the Simpson-Angus Rating Scale were low: mean=12.2 (SD=2.62) in the patients given fananserin and mean=13.5 (SD=4.42) in the patients given placebo (possible range of scores was 10 to 50). During the study, the patients’ scores improved and there was still no difference between treatment groups: mean change from baseline to day 29 was –1.2 in both groups (SD=2.55 in the fananserin group; SD=1.84 in the placebo group).
The five subscores of the Simpson-Angus Rating Scale comprise a sum of items 1 to 6 and individual scores of items 7 to 10. The review of their course during the study revealed nonsignificantly greater worsening of glabellar tap in the placebo group (two [5.9%] of the placebo patients, one [1.6%] of the fananserin patients) (z=1.27, p=0.20, Wilcoxon test approximated by normal distribution). Akathisia worsened in six (9.5%) of the fananserin group but in none of the placebo group (z=–1.54, p=0.12, Wilcoxon test approximated by normal distribution).
Safety Results
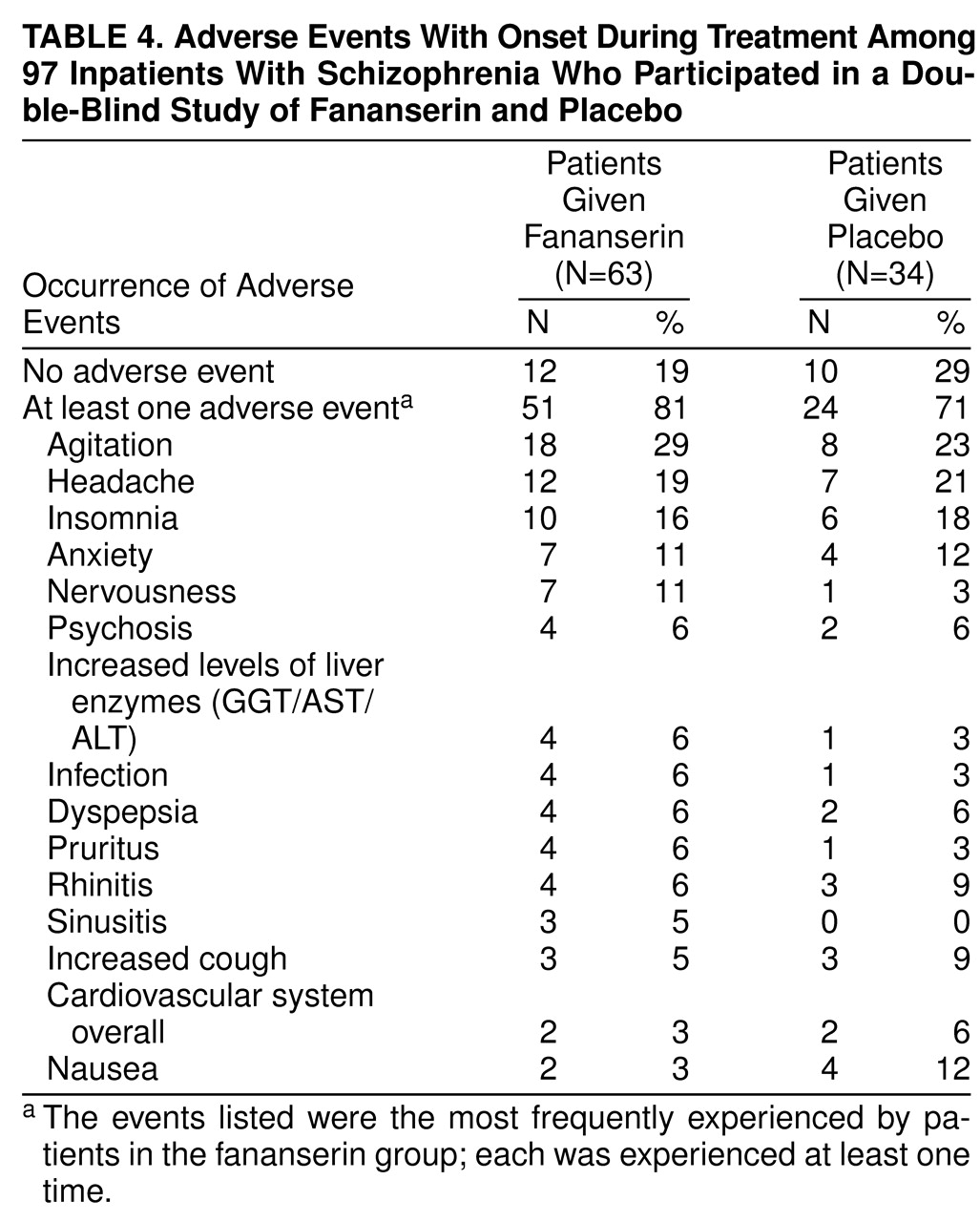
The most frequent adverse events were agitation, headache, insomnia, and anxiety; there was no difference between the two groups in the incidence of these events (
table 4). Nervousness, agitation, and elevated levels of liver enzymes were slightly more frequent in the fananserin group (
table 4).
Because of the α1 affinity of fananserin, the cardiovascular system was carefully monitored in this study. No major abnormality was detected during the study, and no clinically significant increase of QTc, PR, or QRS duration occurred. Moreover, only a few adverse events linked to the cardiovascular system were reported (two patients in each group). Minimum orthostatic hypotension was experienced during the trial (only one adverse event was reported), and values of blood pressure in standing and supine positions did not change significantly during the study.
Pharmacokinetics
At steady-state, which was achieved from day 15, mean trough plasma levels of fananserin ranged from 151 ng/ml (SD=122) to 191 ng/ml (SD=122). To check a possible relationship between concentration and response among patients, the fananserin group was divided into high-plasma-level (≥150 ng/ml) (N=35) and low-plasma-level (<150 ng/ml) (N=28) subgroups according to the trough plasma concentrations at steady-state. The mean plasma levels at day 29 were 70 ng/ml (SD=36) in the low-plasma-level subgroup and 230 ng/ml (SD=119) in the high-plasma-level subgroup. However, the Positive and Negative Syndrome Scale decreases were not grossly different in these two subgroups.
DISCUSSION
The results of this study were consistently unable to show a beneficial effect of fananserin at 500 mg/day over placebo on schizophrenic symptoms. No statistically or clinically significant differences between fananserin and placebo were observed on any measure. None of the measures demonstrated any effect or even a slight trend in favor of fananserin. In addition, the results were homogeneous among the treating facilities. Although results are not secured by a positive control group, it is unlikely that they are due to such methodological flaws as improper dose, duration of treatment, patient population, facility effect, or dropouts.
The 4-week duration of treatment was chosen on the basis of previous trials
(15,
17–
)19, in which an antipsychotic effect was always observed by at least the fourth week. In addition, this duration made it possible to maintain the patients in a hospitalized setting throughout the entire study period, thereby improving compliance.
The study patients were comparable to those studied in other schizophrenia clinical trials
(15,
17,
18). Patients with prolonged hospitalization (more than 3 months) before random assignment to active drug or placebo were not allowed into the study to exclude treatment-refractory patients. Patients were randomly distributed among facilities and treatment groups. They were moderately ill, with symptoms well balanced among the Positive and Negative Syndrome Scale negative symptom, positive symptom, and general psychopathology subscores. Calculations were made for the power of 80%, which is standard for a phase II study.
The overall rate for early discontinuation (below 30%) is quite low when compared with reported rates up to 60% in placebo groups
(17–
19). The early development stage and the new therapeutic target might have been a motivation for keeping patients in the study.
The dose selected (500 mg/day) was based on the highest dose considered to be safe and well tolerated in phase I trials (600 mg/day in patients with schizophrenia). The drug plasma levels were consistent with those found in previous phase I studies (at steady-state with a similar dosing regimen up to 300 mg b.i.d., the mean fananserin trough plasma concentration was 207 ng/ml, SD=126). In addition, there was no difference in efficacy between the patients with low versus high trough plasma concentrations, which could reveal that the dose might have been too low or too high.
The dose was also consistent with rough extrapolations made from experimental data on fananserin affinities for D
4 and 5-HT
2A receptors. Although no selective ligand was available to measure D
4 receptor occupancy directly in vivo, we tried to predict this occupancy from the occupation of 5-HT
2A receptors by fananserin in the rat. The mean median effective dose of fananserin on the rat frontal cortex in vivo was 0.485 mg/kg given orally
(8). The relative ratios of fananserin affinities for the D
4 and the 5-HT
2A receptors varied from 48:1 in rats to 8:1 in humans. Therefore, the blockade of D
4 receptors could be expected at doses ranging from ≅4 mg/kg given orally based on human affinities to ≅25 mg/kg given orally according to animal data.
This estimate was in line with effective doses found in experimental pharmacology models (median effective oral dose range from 0.25 to 48 mg/kg depending on the model and animal) and with the dose tested in this study (500 mg/day for ≅6–10 mg/kg in man).
Although no formal demonstration of the actual in vivo receptor blockade could be performed, and although it is possible that the effective dose is above the dose that causes adverse events, we can assume that an adequate dose range was tested in this study, and it is unlikely that efficacy may have been missed by using too low a dose.
In conclusion, the results of this study do not support the putative therapeutic role of D
4 receptors in schizophrenia
(20). Results with another D
4 antagonist have been reported showing no therapeutic effect and possibly even an aggravation of some positive symptoms
(21). According to these data, it is even possible that worsening of psychosis would have occurred with fananserin if not for the beneficial effects of 5-HT
2A receptor antagonism
(6). Indeed, there is preliminary evidence that MDL-100907, a highly selective 5-HT
2A antagonist, is more effective than placebo in acutely psychotic patients with schizophrenia (personal communication from J. Shipley, June 5, 1997). However, the high 5-HT
2A activity of fananserin did not provide any additional antipsychotic or anxiolytic/antiaggressive effects that could have been expected
(9,
22).
It is worth noting that some patients responded very well to the study treatment, although the treatments were not effective, possibly revealing a placebo effect that is still controversial in schizophrenia
(11). Although a positive control arm in the trial could have helped in our interpretation of the results, this study exemplifies the need to use a placebo control group in antipsychotic studies.
Participating Investigators
R.G. Carter, M.D. (Atlanta); L. Ereshefsky, Pharm.D. (San Antonio, Tex.); L. Fabre, M.D., Ph.D., and J. Rodriguez, M.D. (Houston); J.H. Krystal, M.D., and D.S. Charney, M.D. (New Haven, Conn.); A. Lahti, M.D., and C.A. Tamminga, M.D. (Baltimore); J.P. Lindenmayer, M.D. (New York); M. Plopper, M.D. (San Diego); R. Shelton, M.D., and H.Y. Meltzer, M.D., Ph.D. (Nashville, Tenn.); J.G. Small, M.D., and A. Shekhar, M.D., Ph.D. (Indianapolis); R.G. Stephens, M.D. (Athens, Ga.); S.D. Targum, M.D. (Philadelphia); D.A. Wirshing, M.D., and W.C. Wirshing, M.D. (Los Angeles).