Regional cerebral glucose metabolism, measured by using positron emission tomography (PET) with [
18F]fluorodeoxyglucose (FDG), is a reliable index of neuronal/synaptic activity
(1,
2). Patients with Alzheimer’s disease show heterogeneous low glucose metabolic rates at rest (eyes and ears covered, no sensory stimulation), particularly in the parietal and temporal neocortical association areas
(3), which worsen with dementia progression. In the preclinical and initial stages of Alzheimer’s disease, however, plastic compensatory mechanisms, including synaptic hypertrophy
(4), may help to maintain relatively stable glucose metabolism at rest, suggesting that neurons can perform biochemical activities related to cell survival despite development of Alzheimer’s disease neuropathology
(5,
6) up to a threshold where a dramatic disruption of brain functions is triggered
(5). However, even before the decline of cognitive performance and regional glucose metabolism at rest begins, the accumulation of Alzheimer’s disease neuropathology should impair the metabolic capability of neurons to respond to functional activation
(6,
7). In older Down’s syndrome subjects, who have a 100% risk of developing Alzheimer’s disease, increasing the functional demand on the brain through audiovisual stimulation revealed abnormalities in glucose metabolism before the onset of dementia and despite normal metabolism at rest
(7).
In this study we measured regional cerebral glucose metabolism at rest and during passive audiovisual stimulation in a group of Alzheimer’s disease patients across a wide range of dementia severity. We hypothesized that, as a result of progressive neuronal dysfunction occurring in Alzheimer’s disease, regional cerebral glucose metabolism during neuronal activation would be a more reliable index of dementia severity than metabolism at rest. We focused our analysis on parietal neocortical association areas, which usually are the first and most affected regions in Alzheimer’s disease, and on the auditory and visual cortical areas, which, in contrast, are relatively spared by the disease process
(3,
6,
8) and which have the greatest metabolic increases in response to audiovisual stimulation in older healthy comparison subjects
(9). We expected that, despite the relative preservation of the latter areas, an audiovisual stimulation paradigm that maximizes metabolic demand would reveal a severity-related functional failure in these areas that is not evident in the resting state.
METHOD
The subjects were 15 otherwise healthy Alzheimer’s disease patients, nine men and six women, with a mean age of 70 years (SD=10), a mean education level of 15 years (SD=4), and a mean score on the Mattis Dementia Rating Scale
(10) of 91 (SD=33, range=23–128). They all met the criteria of the National Institute of Neurological Disorders and Stroke and the Alzheimer Disease and Related Disorders Association for “probable” Alzheimer’s disease
(11), and three subjects had histologically confirmed Alzheimer’s disease. They were given neuropsychological tests and PET scans
(3). After complete description of the study, written informed consent was obtained from the patients or their legal guardians (according to protocol 93-AG-193 of the National Institute on Aging institutional review board).
Absolute values for regional cerebral glucose metabolism (in milligrams per 100 g of brain tissue per minute) were determined at rest (with eyes and ears covered) and during audiovisual stimulation by using a Scanditronix PC2048-15B PET scanner, with two sequential injections of FDG during the same PET session, as previously described
(7). During the audiovisual stimulation condition, the patients were required to watch and listen to a colorful movie intended to elicit both sensory and cognitive activation. All of the subjects were presented with the same movie for identical periods of time and were constantly observed to ensure that they complied with the task requirements and did not fall asleep or engage in other cognitive activities
(7).
Regional brain radioactivity levels were determined by using a template of circular regions of interest 8 mm in diameter (48 mm
2), derived from a PET scan of a healthy normal subject. Regions of interest were spaced evenly throughout the cortical ribbon and then grouped into larger anatomical areas within the frontal, limbic, temporal, sensorimotor, parietal, and occipital cortices for a total of 29 regions
(7).
Correlations between dementia severity (as measured by the Mattis Dementia Rating Scale) and cerebral glucose metabolism at rest and during audiovisual stimulation were tested by using regression analysis. Differences between correlation coefficients were assessed by means of t tests for dependent correlations
(12). Statistical significance was taken at p<0.05. To reduce the number of overall statistical tests, we restricted the analysis to the parietal, visual, and auditory cortical regions.
RESULTS
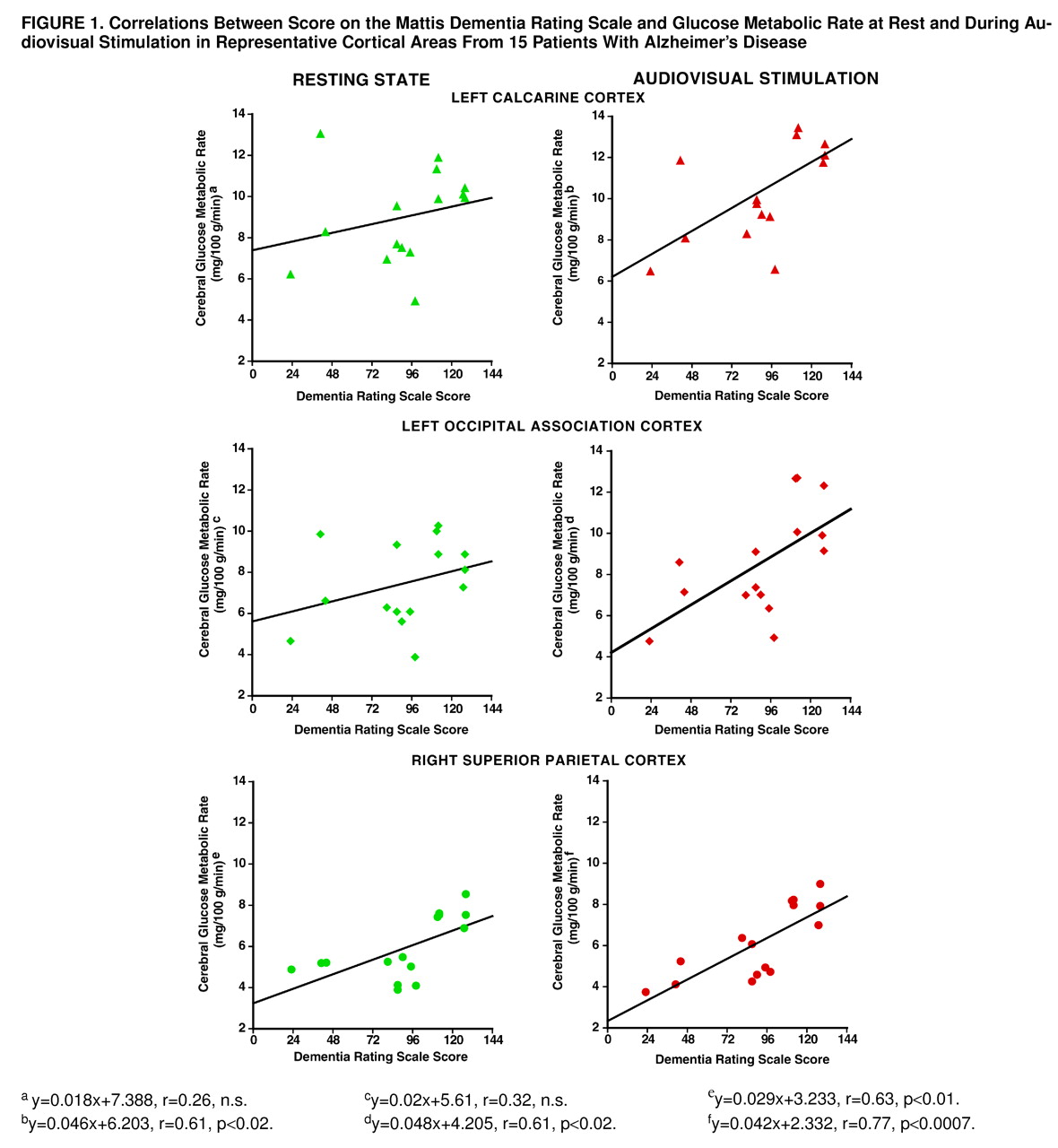
Correlations between dementia severity and resting state glucose metabolism were found bilaterally in the inferior (right: r=0.69, df=13, p<0.004; left: r=0.71, df=13, p<0.003), medial (right: r=0.65, df=13, p<0.009; left: r=0.68, df=13, p<0.005), and superior (right: r=0.63, df=13, p<0.01; left: r=0.82, df=13, p<0.0002) parietal areas (
figure 1). No significant correlation was found between dementia severity and resting metabolic rate in any primary or association visual cortical area or in the superior temporal auditory cortex.
During audiovisual stimulation, dementia severity correlated with glucose metabolism bilaterally in the inferior (right: r=0.75, df=13, p<0.001; left: r=0.68, df=13, p<0.006), medial (right: r=0.53, df=13, p<0.04; left: r=0.62, df=13, p<0.01), and superior (right: r=0.77, df=13, p<0.0007; left: r=0.73, df=13, p<0.002) parietal cortex. In contrast to the resting state, during audiovisual stimulation a significant correlation between dementia severity and metabolic rate also was found in the right (r=0.56, df=13, p<0.03) and left (r=0.61, df=13, p<0.02) visual association cortex and in the left calcarine (r=0.61, df=13, p<0.02) and in the right (r=0.54, df=13, p<0.04) and left (r=0.62, df=13, p<0.02) superior temporal auditory cortical areas (
figure 1). There was no significant relation between age and either test condition for the regions examined.
Correlations with dementia severity were greater during stimulation than at rest for the left calcarine (t=4.6, df=12, p<0.01) and occipital association (t=3.3, df=12, p<0.01) cortical areas but did not differ significantly for other regions.
DISCUSSION
Glucose metabolism in parietal neocortical areas, which are the first and the most affected regions in Alzheimer’s disease
(3,
7), correlated with dementia severity both at rest and during audiovisual stimulation. In contrast, metabolism in the visual and auditory regions, which remain relatively spared by the disease process until its later stages
(3,
6,
8), was correlated with dementia severity during stimulation but not at rest. As regional cerebral glucose metabolic rate is an index of synaptic activity and increases with increasing neuronal firing and rate of stimulation
(2,
13), these findings suggest that the capability of neurons to respond to stimulation is progressively compromised by Alzheimer’s disease
(6). Thus, even in areas with less neuropathological involvement, such as the calcarine cortex, neuronal failure can be demonstrated by increasing the functional demand on the brain. In healthy subjects, stimulation conditions, as compared to resting, also are associated with less metabolic variability, potentially because of more organized brain activity during a specific task
(14).
As the passive audiovisual stimulation in this study required minimal compliance and no task performance
(7), we could study Alzheimer’s disease patients across a wide range of dementia severity. Furthermore, this stimulation was intended to elicit widespread activation of the visual and auditory cortical areas
(9), in order to overcome potential compensatory mechanisms that occur in the plastic reorganization of the brain, at least in the mild to moderate stages of Alzheimer’s disease
(4,
6,
15). Indeed, we recently demonstrated
(6) that neuronal activation in response to patterns of flashing lights in mildly demented subjects is abnormal only at frequencies that elicit the greatest functional demand in healthy comparison subjects, but not with lower frequencies or at rest.
In summary, this study shows that the development of the Alzheimer’s disease neuropathology results in a limitation of the ability of neurons to respond to stimulation and that the degree of functional failure is correlated with the severity of the cognitive impairment. A PET-FDG method with sensory or cognitive stimulation can be used for the in vivo assessment of synaptic integrity and for detecting functional impairment in the preclinical stages of Alzheimer’s disease, aiding early diagnosis of at-risk subjects
(6,
7). Moreover, given that most drugs currently used with Alzheimer’s disease patients act by enhancing cholinergic transmission and thus require viable synapses
(4), this information may be useful in determining which patients are most likely to respond to treatment.