Bipolar disorder (manic-depressive illness) is a chronic episodic disorder with a lifetime prevalence between 0.8% and 1.6%
(1–
3). Over the past several decades, substantial progress has been made in the pharmacologic treatment of the manic phase of bipolar disorder. Numerous authorities and treatment guidelines presently recognize that three individual agents—lithium, valproate, and carbamazepine (typically referred to as mood stabilizers)—and one class of agents—the conventional antipsychotics—have proven efficacy in the short-term treatment of acute mania
(4–
10).
However, it is also well recognized that a substantial proportion of patients with mania fail to respond adequately to these agents, whether they are used alone or in various combinations, or are unable to tolerate them
(9,
10). Moreover, all of these agents are characterized by safety concerns that necessitate regular monitoring (e.g., renal and thyroid toxicity with lithium and tardive dyskinesia and neuroleptic malignant syndrome with typical antipsychotics). Novel medical treatments for the manic phase of bipolar disorder are therefore needed.
The success of the novel antipsychotic agent clozapine for treatment-resistant schizophrenia has resulted in its use for bipolar patients unresponsive to the usual treatment strategies
(11–
15). However, caution must be applied in interpreting these data, since the numbers of patients evaluated in controlled trials were small. Nonetheless, clozapine appears to benefit patients with rapid-cycling illness and those who do not respond to standard treatment. On the other hand, the use of clozapine in bipolar illness is limited by the relatively high incidence of agranulocytosis (annual rate=0.8%), which is tenfold higher than the rate for traditional antipsychotic agents
(16). Furthermore, clozapine has other troublesome side effects (e.g., excessive salivation and sedation) that are likely to limit its use in bipolar patients. Another recently released antipsychotic agent, risperidone, has also been studied in open-label designs, with some studies suggesting a favorable response when it is combined with mood-stabilizing agents
(17,
18). Accordingly, there continues to be a medical need for controlled studies to identify novel pharmacologic agents for use in bipolar disorder.
Olanzapine, a recently introduced novel antipsychotic, demonstrates a pharmacologic profile that most closely resembles that of clozapine
(19). In a series of double-blind controlled trials in patients with schizophrenia and schizoaffective disorder, olanzapine appeared superior to the conventional antipsychotic haloperidol on measures of overall psychotic symptoms, negative symptoms, comorbid mood symptoms, and quality of life
(20–
25). Recent reports of open-label studies suggest that olanzapine may be efficacious in patients with bipolar disorder
(26–
28), including those with manic or mixed symptoms inadequately responsive to lithium, anticonvulsants, and typical antipsychotics. Thus, on the basis of these reports and the findings of mood stabilization with olanzapine among both depressed schizophrenic patients
(20) and schizoaffective bipolar patients
(29), we decided to study olanzapine’s efficacy in the treatment of acute manic and mixed episodes in bipolar I disorder with and without psychotic features. We report on a double-blind study that included 139 patients and was conducted in the United States at 16 study sites between October 1996 and August 1997.
METHOD
Patients between the ages of 18 and 65 years were enrolled in the study. All study subjects met the DSM-IV criteria for bipolar disorder, either manic or mixed episode (with or without psychotic features), on the basis of the Structured Clinical Interview for DSM-III-R (SCID)
(30). Manic or mixed episodes were of at least 2 weeks’ duration. A minimum total score of 20 on the Young Mania Rating Scale
(31) was required. Patients were excluded if any of the following criteria were met: serious, unstable illness such that hospitalization was anticipated within 3 months or death was anticipated within 3 years; DSM-IV-defined substance dependence (except nicotine or caffeine) within the past 3 months; and serious risk of suicide. The study was fully explained to each patient, and written informed consent was obtained before their participation in the study.
This was a randomized, double-blind, placebo-controlled parallel group study. Severity of illness and psychopathology was measured by the following rating scales: the Young Mania Rating Scale
(31), the 21-item Hamilton Depression Rating Scale
(32), the Positive and Negative Syndrome Scale
(33), and the Clinical Global Impression (CGI), Bipolar Version
(34). Quality of life was assessed with the Medical Outcomes Study 36-item Short-Form Health Survey (SF-36)
(35,
36) Change from baseline to endpoint in total score on the Young Mania Rating Scale was the primary efficacy measure. Interrater reliability assessments with the Young Mania Rating Scale were conducted before the study began by measuring the correlation of each rater’s scoring with the groupwise median score for each item. The correlation coefficients ranged between 0.76 and 0.99, with a median of 0.94. Extrapyramidal symptoms were assessed with the Simpson-Angus Rating Scale
(37), the Barnes Akathisia Scale
(38), and the Abnormal Involuntary Movement Scale
(39).
After a 2- to 4-day washout period of all medications except benzodiazepines, qualified patients were randomly assigned to one of two treatment groups, olanzapine or placebo, in a 1:1 ratio. Seventy patients were assigned to olanzapine and 69 to placebo. To ensure patient safety, a minimum of 1 week of hospitalization was required. After 1 week, patients with a CGI, Bipolar Version, severity of mania score of 3 or lower and a reduction of 50% or more in Young Mania Rating Scale total score could be discharged, if clinically appropriate.
Double-blind acute therapy lasted for 3 weeks. Patients began double-blind therapy with either olanzapine, 10 mg (two 5-mg tablets), or placebo (two placebo tablets), given once per day, preferably in the evening. After the first day of treatment, the daily dose could be adjusted upward or downward, as clinically indicated, by 5-mg increments/decrements within the allowed dosage range of 5–20 mg/day. Decreases in dosage because of adverse events could occur at any time by any number of one-tablet (5-mg) decrements (at the investigator’s discretion), to a minimum of one tablet per day.
Use of concomitant medications was limited to lorazepam, up to 4 mg/day, if necessary, to alleviate severe agitation during the first 7 days of therapy; then, during the next 3 days, 2 mg/day could be used. Also, benztropine, up to a maximum dose of 2 mg/day, could be used for treatment-emergent extrapyramidal symptoms. The prophylactic use of benztropine for extrapyramidal symptoms was not allowed.
A total of 139 patients were enrolled in the study; 70 were randomly assigned to olanzapine and 69 to placebo. The treatment groups did not significantly differ with respect to baseline patient characteristics or severity of illness ratings. Patients were generally in their late 30s (mean age=39.5 years, SD=11.0), the majority (72.7%) were Caucasian, and half (51.8%) were male. The majority of the patients (82.7%) were experiencing a manic episode, while the rest (17.3%) were experiencing a mixed episode. Overall, 53.2% displayed psychotic features. Of those with psychotic symptoms, 85.1% displayed mood-congruent psychotic features. A DSM-IV-defined rapid-cycling course was present in 32.4% of the patients. Comparisons of the treatment groups at baseline on efficacy variables demonstrated no evidence of any statistically significant differences.
All statistical analyses were done on an intent-to-treat basis; that is, data on all randomly assigned patients were included in the analysis. Patients’ data were included in the analysis of change if they had both a baseline and a postbaseline observation. For the analysis of baseline efficacy and extrapyramidal symptom scores, only the patients with a baseline and a postbaseline observation were included, to be consistent with the analysis of change from baseline. All patients randomly assigned to double-blind therapy were included in the analysis of baseline patient and illness characteristics as well as the analysis of the incidence of treatment-emergent adverse events. Total scores from rating scales were derived from the individual items; if any single item was missing, the total score was treated as missing.
Analysis of variance (ANOVA) was used to evaluate the continuous efficacy and safety variables; the models included the terms for treatment, investigator, and treatment-by-investigator interaction. Data were pooled from investigators who did not have at least two patients per treatment. Fisher’s exact test, two-tailed, was used to analyze treatment effects for categorical efficacy and safety variables. For selected efficacy variables, weekly changes from baseline were analyzed. The protocol established the primary efficacy analysis as the mean change from baseline to endpoint (last observation carried forward) in Young Mania Rating Scale total score.
All cited p values were two-tailed, with a significance level of 0.05 as specified in the protocol. SAS
(40) was used for all analyses.
RESULTS
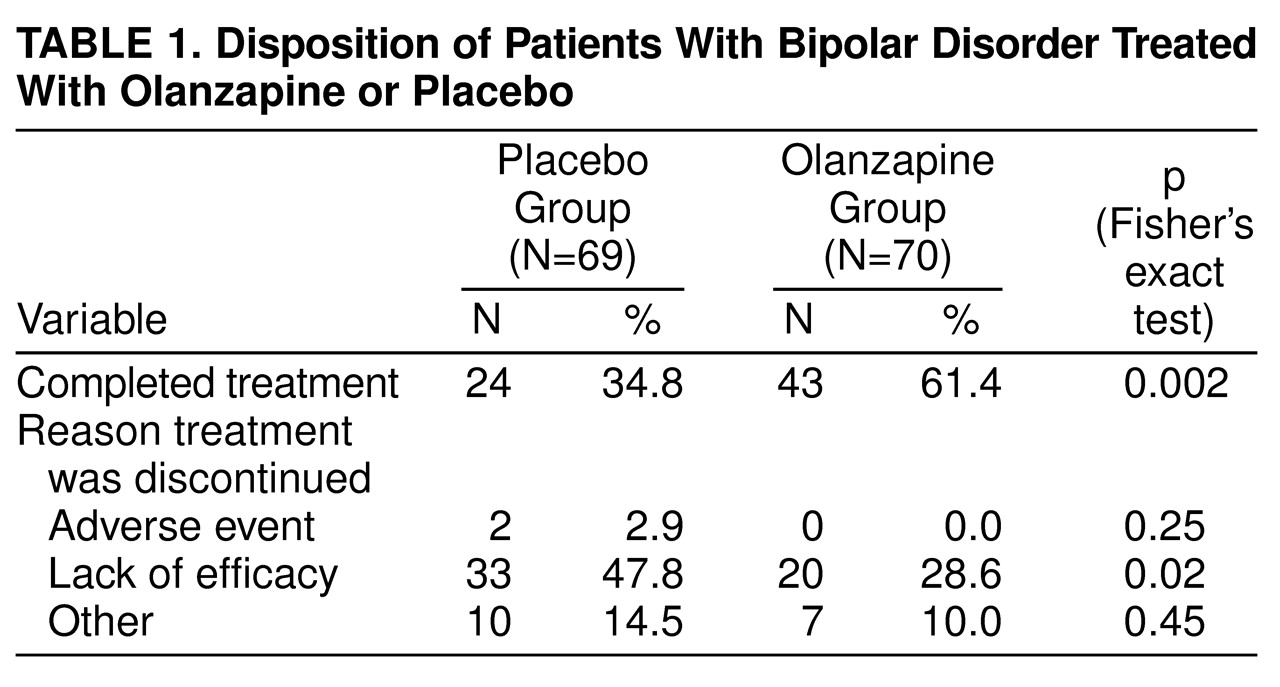
The disposition of the study group during the acute phase of the trial is shown in
table 1. Almost twice as many olanzapine-treated patients as placebo-treated patients completed the acute phase of the study. Nearly one in two patients receiving placebo had to discontinue because of lack of efficacy, compared to one in four olanzapine-treated patients.
The mean modal and median modal doses of olanzapine were 14.9 mg/day (SD=5.0) and 15 mg/day, respectively. The modal dose was defined as the daily dose most often taken.
There were no statistically significant differences between the two treatment groups in the number of patients who used anticholinergic medication, benzodiazepines, or any other concomitant medications during the study. Of all patients, 86.3% (N=120) took at least one dose of a benzodiazepine, and 9.4% (N=13) took at least one dose of an anticholinergic medication. Of the patients receiving benzodiazepines, the placebo-treated patients received a significantly greater mean daily dose than the olanzapine-treated patients (placebo: mean=1.7 mg/day, SD=1.3; olanzapine: mean=1.1 mg/day, SD=1.2; F=10.42, df=1, 102, p=0.002). There was no significant difference between the two groups in mean daily dose of anticholinergics.
Efficacy
Endpoint analysis
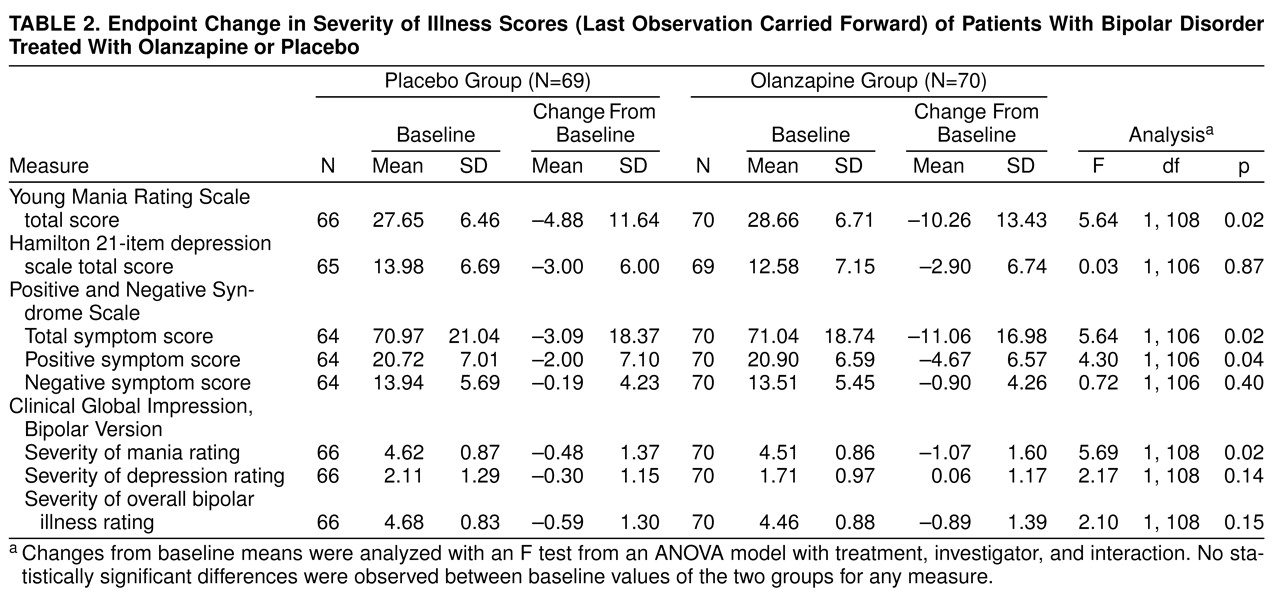
Mean change from baseline to endpoint (last observation carried forward) of the 3-week acute phase was used to compare the efficacy of the two treatments (
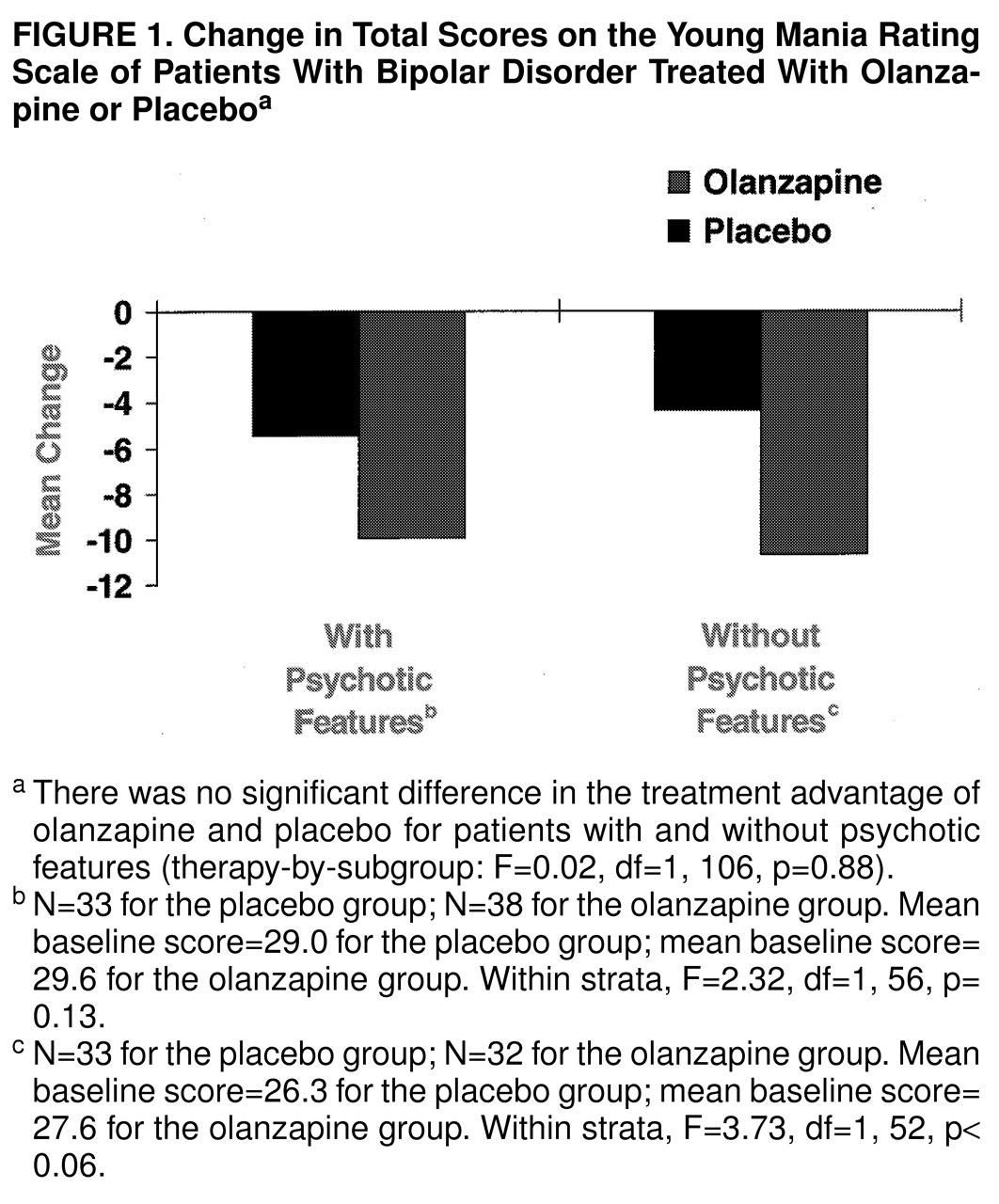
table 2). In the primary efficacy analysis, change from baseline to endpoint in total scores on the Young Mania Rating Scale, the olanzapine group experienced significantly greater mean improvement than the placebo group. The mean difference between the olanzapine and placebo groups was –5.38 points (95% confidence interval=–10.31 to –0.93). Subgroup analyses were performed on the basis of the presence or absence of psychotic features and the presence or absence of a rapid-cycling course as well as between patients with pure and mixed mania. When patients with and without psychotic features were compared (
Figure 1), there was no difference in the treatment advantage of olanzapine relative to placebo (F=0.02, df=1, 106, p=0.88). The olanzapine-treated patients with a rapid-cycling course showed a significantly greater mean change from baseline in Young Mania Rating Scale total scores (mean=–13.89, SD=7.97) than did the placebo-treated patients (mean=–4.12, SD=11.45) (F=7.25, df=1, 35, p=0.01). There was no significant difference in mean change from baseline between the patients with pure and mixed mania who were treated with olanzapine (mean=–10.84, SD=13.72, and mean=–7.42, SD=12.06, respectively; F=0.01, df=1, 55, p=0.91).
Analyses of scores on the secondary efficacy scales were also performed. The olanzapine patients had a significantly greater mean decrease from baseline in CGI, Bipolar Version, severity of mania scores compared with the placebo patients (
table 2). They also had a significantly greater reduction in total scores and positive symptom scores on the Positive and Negative Syndrome Scale.
As expected with the relatively low baseline values in both treatment groups, the analysis of mean change from baseline to endpoint in Hamilton depression scale total scores showed no significant treatment advantages for improvement of depressive symptoms (
table 2). Olanzapine was not associated with a significant baseline-to-endpoint worsening in Hamilton depression scale total score relative to placebo. There was also no difference in mean change from baseline in Hamilton depression scale total scores between patients with pure and mixed mania who were treated with olanzapine (mean=–2.95, SD=6.10, and mean=–2.67, SD=9.55, respectively; F=0.14, df=1, 54, p=0.71).
Weekly analysis
The olanzapine group consistently showed greater mean improvement in total scores on the Young Mania Rating Scale; the difference was statistically significant at week 3 (F=5.64, df=1, 108, p=0.02). For the olanzapine-treated patients, 81% of the last-observation-carried-forward improvement in mean Young Mania Rating Scale total score was achieved after the first week of treatment. Visitwise analyses of the CGI, Bipolar Version, ratings of overall severity of illness and severity of mania and the Positive and Negative Syndrome Scale total, positive, and negative symptom scores were also performed. At weeks 2 and 3, the olanzapine group demonstrated significantly greater improvement relative to the placebo group in severity of mania (F=4.36, df=1, 108, p=0.04, and F=5.69, df=1, 108, p=0.02, respectively) and in Positive and Negative Syndrome Scale total scores (F=5.30, df=1, 106, p=0.02, and F=5.64, df=1, 106, p=0.02, respectively), and at week 3 in Positive and Negative Syndrome Scale positive scores (F=4.30, df=1, 106, p=0.04). In comparison with the olanzapine group, the placebo group did not show significantly greater mean improvement on any scale at any visit.
Response analysis
The protocol defined a responder a priori as any patient achieving a 50% or greater decrease in total score on the Young Mania Rating Scale from baseline to endpoint. Significantly more patients responded to olanzapine than to placebo (olanzapine: N=34 of 70, 48.6%; placebo: N=16 of 66, 24.2%; p=0.004, Fisher’s exact test).
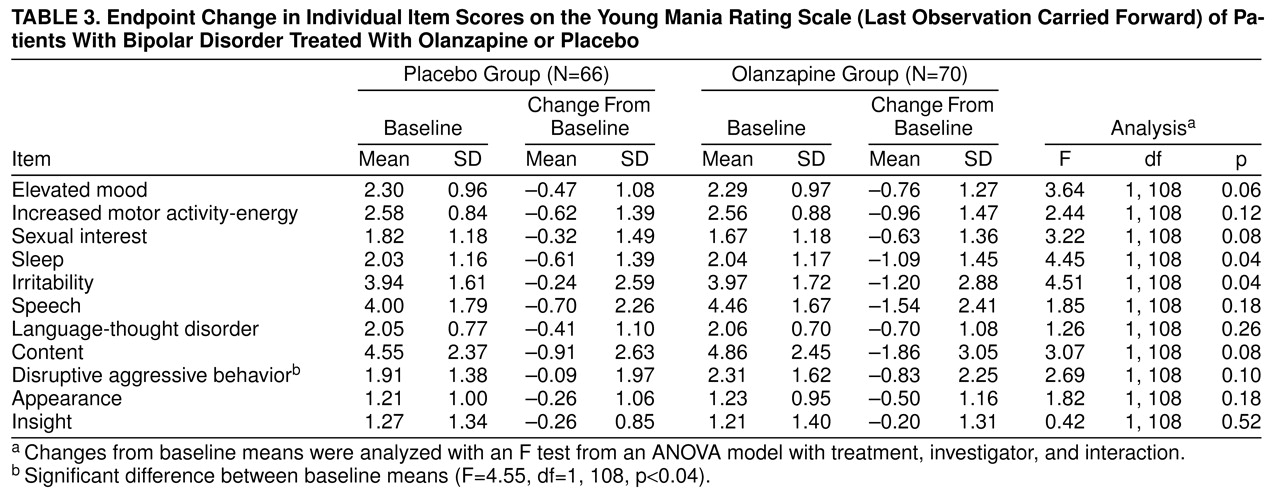
Young Mania Rating Scale individual items
The olanzapine group demonstrated a numerically greater mean improvement relative to the placebo group on all items except insight (
table 3). Importantly, the difference for elevated mood nearly reached statistical significance. The olanzapine group’s greater mean improvement in the sleep and irritability items did achieve statistical significance.
Quality of life
In nine of the 10 components of the SF-36 there was no statistically significant difference between the two groups over the 3-week acute phase. The olanzapine-treated patients had significantly greater improvement in their physical functioning than the placebo-treated patients, as measured by the SF-36 physical functioning subscore (olanzapine: mean=4.01, SD=13.27, placebo: mean=–1.84, SD=14.50; F=6.06, df=1, 94, p=0.02).
Safety
Treatment-emergent adverse events (events that first appeared or worsened during double-blind therapy)
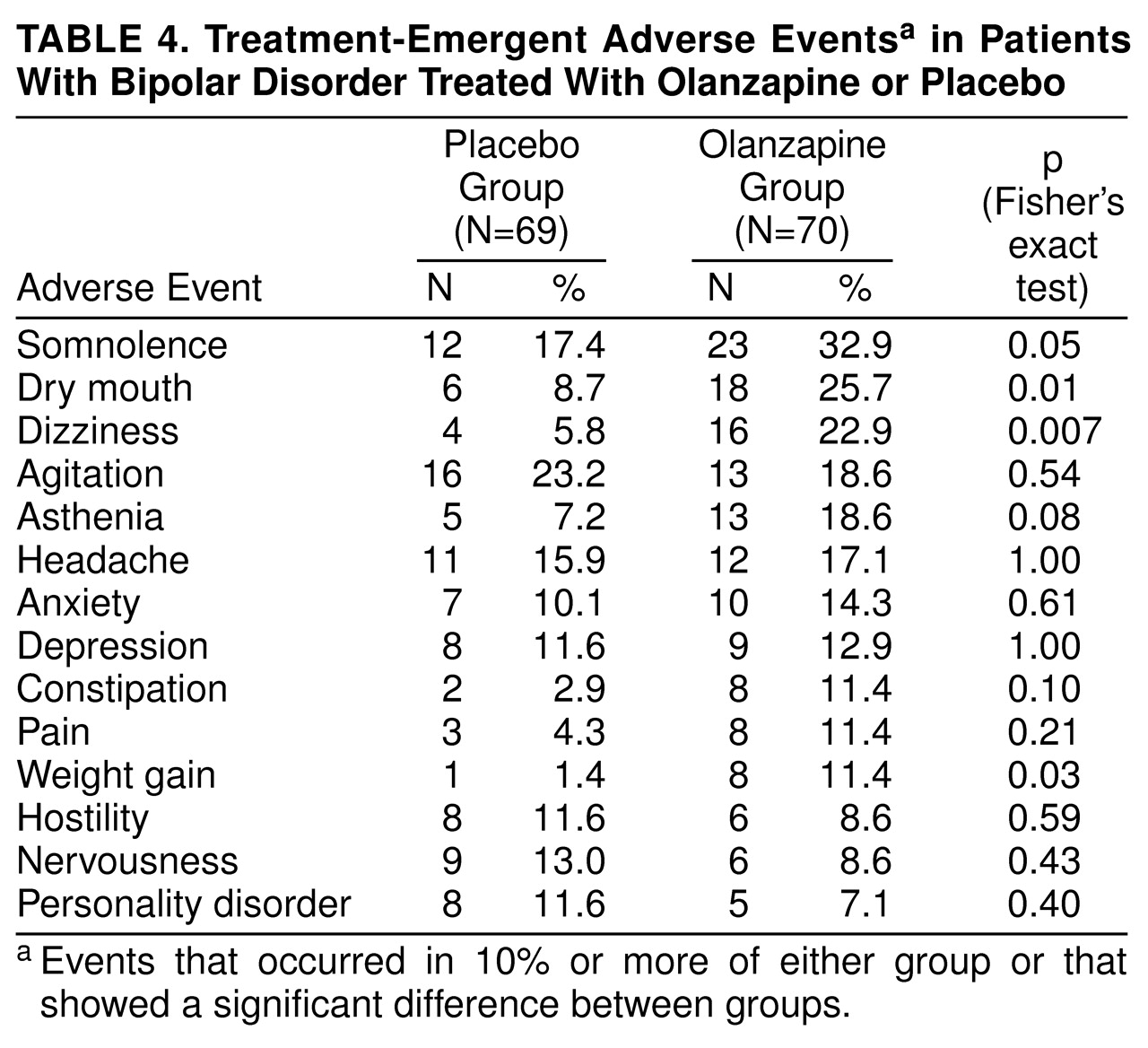
Somnolence, dry mouth, dizziness, and weight gain occurred significantly more frequently in the olanzapine group than in the placebo group (
table 4). However, no olanzapine-treated patients discontinued therapy because of an adverse event, whereas two placebo-treated patients discontinued (one because of convulsions and one because of dystonia).
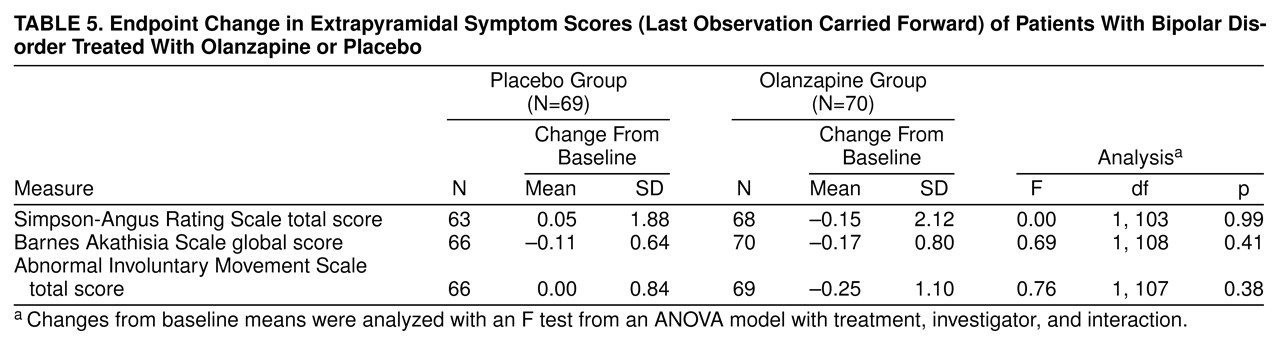
Extrapyramidal symptoms
Extrapyramidal symptoms were infrequent among the study participants, and thus anticholinergic use was negligible in both treatment groups.
Table 5 shows that pseudo parkinsonism (Simpson-Angus scale), akathisia (Barnes scale), and dyskinesias (Abnormal Involuntary Movement Scale) were not significantly different between the two groups with respect to mean change from baseline to endpoint.
Vital signs and weight
No clinically significant changes in vital signs were observed in either treatment group. However, olanzapine-treated patients on average gained 1.65 kg (SD=2.54) of weight from baseline to endpoint, whereas there was a mean weight loss of 0.44 kg (SD=2.35) among the placebo-treated patients (F=11.72, df=1, 101, p<0.001). No patients discontinued therapy because of an adverse event associated with weight.
Laboratory values
No clinically significant changes in laboratory values were observed in either treatment group. In the analysis of treatment-emergent laboratory values at any time during the acute phase, the occurrence of increased ALT/SGPT values at any time was significantly different between the two groups (olanzapine: 17.6%; placebo: 0%; p<0.001, Fisher’s exact test). However, all of the ALT/SGPT values returned to normal during continued treatment with olanzapine, and none of the patients displayed clinical symptoms of hepatic dysfunction at any time, consistent with observations from earlier studies with olanzapine
(24).
ECG
There were no significant differences between the groups in the analysis of mean change from baseline to endpoint in ECG heart rate and interval times.
DISCUSSION
This randomized, placebo-controlled, double-blind study suggests that olanzapine is effective in the treatment of acute mania, as evidenced by the decreases in total scores on the Young Mania Rating Scale; in severity of mania ratings on the CGI, Bipolar Version; and in total and positive symptom scores on the Positive and Negative Syndrome Scale. Also, the percentage of patients who discontinued treatment because of lack of efficacy was significantly smaller (p=0.02) in the olanzapine group (28.6%) than in the placebo group (47.8%). That olanzapine may have antimanic effects is further suggested by the magnitude of response to treatment as illustrated by the responder analysis, where 48.6% of the olanzapine-treated patients had an improvement of 50% or more in Young Mania Rating Scale total score, compared with 24.2% of the placebo-treated patients. Similar degrees of response were observed in two recent clinical trials in the treatment of mania
(41,
42). Bowden and collaborators
(41) found that 48% of divalproex-treated patients and 49% of lithium-treated patients, compared with 25% of placebo-treated patients, had an improvement of at least 50%. However, those investigators used the manic syndrome subscale of the Schedule for Affective Disorders and Schizophrenia
(43) instead of the Young Mania Rating Scale to assess manic symptoms. Pope and collaborators
(42), using the Young Mania Rating Scale to assess manic symptoms, found that 54% of valproate-treated patients improved at least 50%. Considering that there may be differences in study populations and efficacy measures across studies, the most appropriate study would be a head-to-head comparison trial of olanzapine against other mood-stabilizing drugs.
Regarding individual manic signs and symptoms, as shown on
table 3, olanzapine was nearly significantly superior to placebo for the two Young Mania Rating Scale items that assess mood symptoms and significantly superior for irritability. It has been reported that conventional antipsychotics induce depressive symptoms when they are used in the maintenance treatment of bipolar disorder
(44,
45). This induction of depressive symptoms limits conventional antipsychotic use in bipolar disorder, especially in maintenance treatment. In our acute treatment study, Hamilton depression scale total scores and CGI, Bipolar Version, severity of depression scores showed no significant differences in depressive symptoms between the olanzapine and placebo groups, suggesting that olanzapine does not have depressogenic effects in the short-term treatment of acute mania in bipolar disorder
(46). In a recent study comparing olanzapine and haloperidol in patients with schizophrenia and comorbid depressive symptoms
(20), olanzapine-treated patients showed significant improvement in depressive symptoms in comparison with haloperidol-treated patients. However, whether olanzapine has antidepressant or depression-inducing effects in bipolar disorder can only be ascertained in controlled maintenance studies.
Since olanzapine has antipsychotic properties, it is not surprising that it demonstrated superior efficacy for psychotic symptoms as measured by the positive symptoms subscale of the Positive and Negative Syndrome Scale. Moreover, the antimanic response to olanzapine, as measured by the Young Mania Rating Scale total score, was similar in nonpsychotic and psychotic patients.
Use of conventional antipsychotic agents in the treatment of mania remains controversial. Although their efficacy is well-documented
(46–
50), concerns regarding a possible higher risk of extrapyramidal symptoms and tardive dyskinesia have been raised
(51). Despite this risk, conventional antipsychotics are widely used, as documented in a recent pharmacoepidemiologic study
(8) which showed that up to 89% of manic inpatients and 64% of outpatients were taking conventional antipsychotics alone or in combination with other agents. However, in our study no difference between olanzapine and placebo in terms of extrapyramidal adverse effects was found. Furthermore, studies of patients with schizophrenia treated with olanzapine show a small risk of developing extrapyramidal symptoms
(52) or tardive dyskinesia
(53). However, these studies were conducted with schizophrenic patients, and the findings may not extrapolate to patients with bipolar disorder. Further studies and clinical experience will help to clarify the role of olanzapine in the development of tardive dyskinesia in bipolar patients.
In this study, no olanzapine-treated patients withdrew from the study because of a serious adverse event. Very few extrapyramidal symptoms emerged, and use of anticholinergic medication was negligible for both treatment groups. For all patients there were no clinically significant changes in vital signs, laboratory analyses, ECGs, or extrapyramidal symptoms. Although no patients in this study discontinued treatment with olanzapine secondary to weight gain, a mean weight gain of 1.65 kg was observed in a 3-week period; thus, it is important for the treating clinician to monitor and address weight on an ongoing basis.
There were several limitations of the study. 1) The duration of the acute phase of the study was limited to 21 days. However, because of ethical concerns and the severe symptoms of manic patients, extending the trial beyond 3 weeks would not follow current clinical practice. Other published studies of treatment of the acute mania of bipolar disorder
(41,
42) also used 21 days for the length of treatment; therefore, this treatment period appears to be a reasonable time interval to balance measuring the clinical effects of the study drug and the ethical concerns of treating patients suffering an acute manic episode with placebo. 2) There was a high dropout rate among the placebo-treated patients. Again, for ethical reasons, patients were allowed to discontinue the double-blind acute phase after 1 week in order to start open-label olanzapine. This may have contributed to the high dropout rate, which occurred for 65.2% of the placebo-treated patients, compared with 38.6% of the olanzapine-treated patients. Similarly, in the study by Bowden et al.
(41), the dropout rates were 48% for divalproex, 61% for lithium, and 64% for placebo. On the other hand, in a similarly designed study
(42), the dropout rates were 76% for valproate and 79% for placebo. 3) No information on the use of olanzapine compared with placebo in maintenance treatment was provided by this study. Further studies are in progress to determine the role of olanzapine in long-term treatment of bipolar disorder. 4) Patients in our study experienced a high rate of response to placebo (24.2%). This response rate was similar to the 25% placebo response found by Bowden and et al.
(41), who compared divalproex, lithium, and placebo, and by Janicak et al.
(54), who compared clonidine and placebo, in the acute treatment of mania; however, it was significantly higher than the 5% placebo response rate reported by Pope and colleagues
(42). It is possible that the patients who did not respond to lithium who were included in the study by Pope et al. were less likely to respond to placebo. A possible explanation for the high response to placebo in our study is the use of lorazepam, which has been reported to have antimanic effects
(55). However, the dose allowed in our study was similar to that in the Pope et al. study (4 mg/day up to day 10). In contrast, in the Bowden et al. study, where there was a similar placebo response (25% of subjects), lorazepam was allowed at lower doses (2 mg/day up to day 4 and then 1 mg/day on days 5–10). Another possible explanation for the high response to placebo is the study requirement of inpatient treatment for at least 1 week. Hospitalization in itself provides treatment by reducing the level of sensory stimulation under the care of health professionals
(6). Thus, even part of the olanzapine-treated patients’ improvement could be attributed to the structured environment provided by participation in an inpatient clinical trial. It is possible that in a small number of patients, recovery was part of the natural course of illness, which may explain a portion of the placebo response.
Although the primary rating scale, the Young Mania Rating Scale, showed significantly different results with olanzapine and placebo, significant differences were not observed in the results on all the scales. Importantly, no differences were found in Hamilton depression scale scores and in CGI, Bipolar Version, severity of depression ratings, suggesting that unlike other antipsychotic agents, olanzapine does not induce depressive symptoms. It is not surprising that there were no between-group differences in negative symptom scores on the Positive and Negative Syndrome Scale, since negative symptoms are not generally observed in bipolar patients. On the other hand, significant differences were observed in the Positive and Negative Syndrome Scale total and positive symptom scores.
In summary, this study suggests that olanzapine is effective in the treatment of acute mania. Also, olanzapine was well tolerated, with no dropouts due to adverse events. More clinical experience and controlled studies in acute and maintenance treatment are needed to clarify further the role of olanzapine in the treatment of bipolar disorder.
ACKNOWLEDGMENTS
The following institutions and individuals, in addition to the authors, participated in the Olanzapine HGEH Study Group: Washington Clinical Research Center, Neuro-Psychiatric Services, Greater Washington, Falls Church, Va. (25 patients), David G. Daniel, M.D.; VA Medical Center, Dallas (17 patients), Frederick Petty, M.D., Ph.D.; Stanley Center for Treatment of Bipolar Disorders, Western Psychiatric Institute and Clinic, University of Pittsburgh Medical Center (12 patients), K.N. Roy Chengappa, M.D.; the Department of Psychiatry, Harvard Medical School, McLean Hospital, Belmont, Mass. (11 patients), Franca Centorrino, M.D.; the Biological Psychiatry Program, University of Cincinnati College of Medicine (10 patients), Susan L. McElroy, M.D., and Paul E. Keck, M.D.; Milwaukee Psychiatric Hospital (10 patients), Richard Wang, M.D., Ph.D.; the Department of Psychiatry and Behavioral Sciences, The University of Texas Medical Branch at Galveston (nine patients), James Russell, M.D.; Bellevue Hospital, New York University Medical Center (eight patients), Norman Sussman, M.D.; The Psychiatric Institute, Department of Psychiatry, University of Illinois, Chicago (six patients), Philip G. Janicak, M.D.; Gracie Square Hospital, New York (six patients), Robert Levine, M.D.; the Department of Psychiatry, Emory University of School of Medicine (six patients), Charles B. Nemeroff, M.D., and Emilie D. Risby, M.D.; University of Arizona, Arizona Health Services Center, Tucson (five patients), Alan J. Gelenberg, M.D.; the Clinical Neurology Pharmacy Research Program, Medical University of South Carolina, Charleston (five patients), S. Craig Risch, M.D.; the Department of Psychiatry, VA Medical Center, University of California at Los Angeles (four patients), Lori Altshuler, M.D.; University of Texas Mental Sciences Institute, Houston (three patients), Alan C. Swann, M.D.; and Rush Institute for Mental Well-Being, Chicago (two patients), Jan Fawcett, M.D.