Cocaine abuse continues to be a major public health concern and is an important cofactor in the AIDS epidemic
(1,
2). Cocaine use may be associated with cardiac infarcts, cerebral infarcts, depression, and neuropsychological abnormalities
(3–
6). However, the underlying pathophysiological mechanisms responsible for many of these complications are not well understood. In addition, neurochemical abnormalities reflecting brain injury, such as increased total creatine and myoinositol, in cocaine users may be subclinical and have been reported in asymptomatic individuals
(7). Studies have also found that cocaine-dependent women had fewer perfusion abnormalities in the frontal lobe than cocaine-dependent men
(8) and that female cocaine users had better treatment outcomes than male cocaine users
(9,
10). Therefore, it is important to investigate whether cocaine use causes persistent cerebral abnormalities and whether such abnormalities are affected by gender. This information may provide insight into the factors that affect the brain’s vulnerability to the harmful effects of cocaine.
Previous neuroimaging studies in cocaine users showed a variety of long-term functional deficits. Positron emission tomography (PET) studies in detoxified cocaine users found reduced local cerebral metabolic rates for glucose utilization in the frontal and subcortical brain regions
(11–
13), down-regulation of postsynaptic dopamine receptor binding with recovery after a drug-free interval
(14), and reduced dopamine release in the striatum but increased dopamine release in the thalamus
(15). Both PET and single photon emission computed tomography (SPECT) studies in cocaine users have shown generalized cerebral hypoperfusion
(16–
20), particularly in the frontal cortex
(12). One SPECT study
(8) specifically addressed gender differences and found that cocaine-dependent women had fewer perfusion abnormalities in the frontal lobe than cocaine-dependent men. These neuroimaging studies demonstrate that brain abnormalities associated with cocaine abuse may be persistent and may vary with gender.
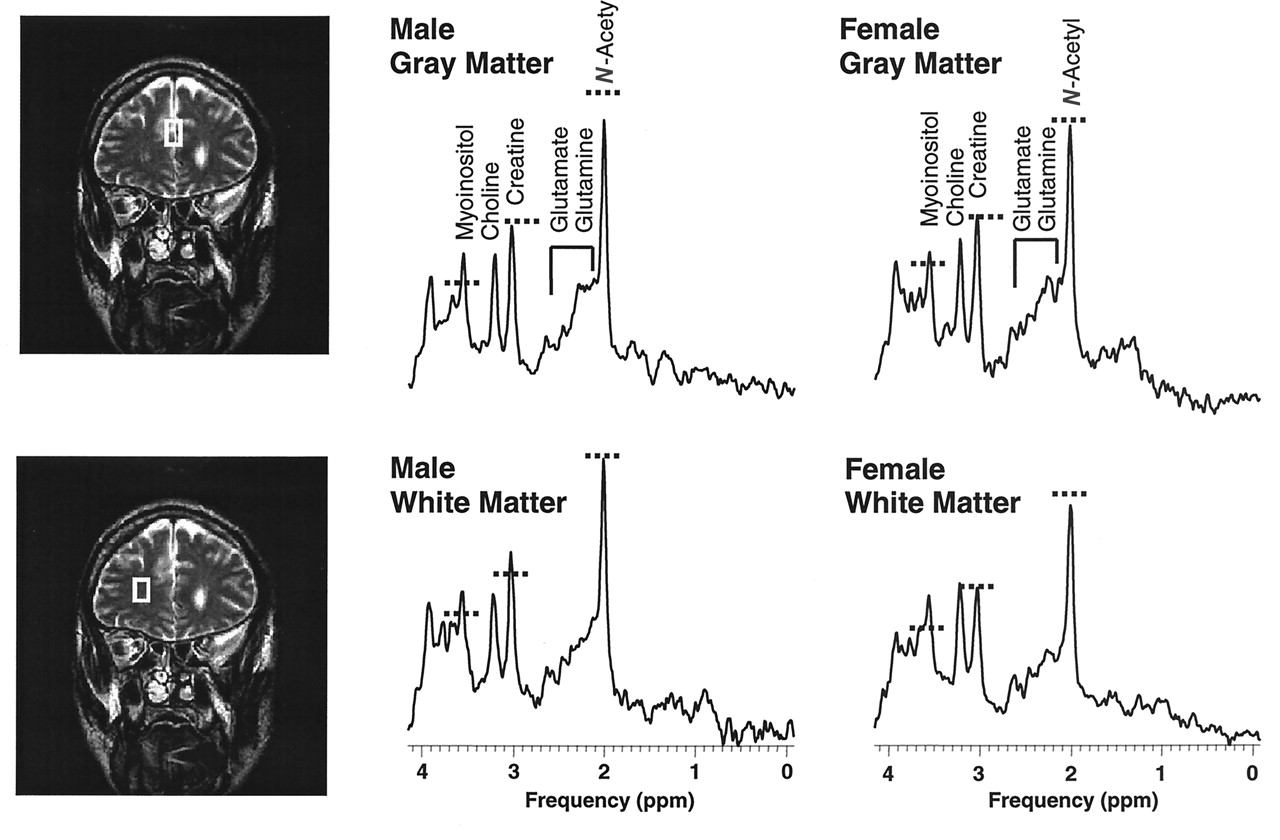
Proton magnetic resonance spectroscopy (
1H-MRS) offers an opportunity to evaluate in vivo neurochemical changes that might occur in cocaine users. With
1H-MRS one can noninvasively measure several cerebral metabolites. Examples of these metabolites include
N-acetylaspartate, a putative neuronal marker
(21); glutamate and glutamine, excitatory neurotransmitters (glutamate also may co-localize with
N-acetylaspartate as
N-acetylaspartylglutamate
[22]); creatine and phosphocreatine, measures of energy metabolism; choline-containing compounds, cell membrane constituents; and myoinositol, a glial marker
(23). Recent technical advances allow the acquisition of high-quality
1H spectra from the frontal lobe
(24), an area that was previously considered unsuited for
1H-MRS but is of great importance for drug abuse research. Only a few MRS studies
(7,
25,
26) have evaluated cocaine users for cerebral metabolite abnormalities; however, these studies did not examine the frontal brain region or look for possible gender effects in acute or chronic cocaine use. The aims of this study were to evaluate persistent cerebral metabolite abnormalities in the frontal lobe of asymptomatic, abstinent cocaine users and to determine whether male cocaine users are affected differently than female users.
METHOD
Sixty-four abstinent (>5 months) chronic cocaine users (34 male and 30 female) aged 20–39 years (mean=31.6 years, SD=4.0) and 58 normal comparison subjects without a history of drug use (29 male and 29 female) aged 18–39 years (mean=30.5 years, SD=5.2) were consecutively recruited and studied with magnetic resonance imaging (MRI) and localized 1H-MRS. The cocaine users were recruited from several local drug rehabilitation centers. The inclusion criteria for the cocaine users were 1) age 18–39 years; 2) DSM-IV criteria for cocaine dependence met; 3) crack cocaine the primary form of cocaine use; 4) more than 5 months’ abstinence from cocaine; 5) a screening evaluation showing normal results of physical and neuropsychiatric examinations; 6) negative HIV-1 serology; and 7) a negative urine toxicology screen (cocaine, amphetamine, marijuana, benzodiazepines, barbiturates, and opiates) both at initial screening and on the day of the MRI/MRS. In addition, subjects in both groups were excluded if they had 1) a history of alcohol abuse or dependence or other substance dependence (except for cocaine in the cocaine user group, nicotine, and caffeine); 2) current or previous chronic medical or neuropsychiatric illnesses (e.g., diabetes, hypertension, stroke, seizure disorder, schizophrenia, or major depression except during the drug withdrawal period); 3) substantial abnormalities in routine blood cell counts, chemistries, serum creatine and creatinine levels, thyroid panel, HIV-1 serology, syphilis serology, and hemoglobin electrophoresis; 4) head trauma with loss of consciousness; 5) MRI showing major structural abnormalities (e.g., cortical infarcts, arteriovenous malformations, or tumors); 6) pregnancy in female subjects (screened by urine pregnancy tests); and 7) any metallic objects in the body. Each subject also underwent a detailed interview for cocaine use and history of other drug use, as well as a battery of neuropsychological tests (results not shown). Before the study each subject was verbally informed of the study protocol and signed an informed consent form approved by the institutional review board at Harbor–UCLA Research and Education Institute.
All subjects underwent brain MRI to assess structural abnormalities. MRI and 1H-MRS were performed on a 1.5-T scanner (Signa 5.4, General Electric, Milwaukee). The examination began with the acquisition of a sagittal T1-weighted localizer (TE=11 msec, TR=500 msec, 4-mm slice thickness, 1-mm gap, 24-cm field of view) followed by an axial fast inversion recovery scan (TE=32 msec, TI=120 msec, TR=4000 msec, 3.5-mm slice thickness, no gap, 24-cm field of view). MRS voxels were defined from a coronal fast double spin echo sequence (TE1=17 msec, TE2=102 msec, TR=4000 msec, 5-mm slice thickness, no gap, 24-cm field of view).
MRS was performed in the midfrontal gray matter and the right frontal white matter. Voxel sizes ranged between 3 and 5 cc, depending on the individual anatomy of the subject; voxel sizes and locations were carefully chosen to ensure that each voxel contained primarily gray or white matter and were placed in normal-appearing brain regions. Data were acquired with the use of an optimized double spin echo sequence, point resolved spectroscopy (PRESS)
(24,
27) (TE=30 msec, TR=3000 msec, 128 averages, 2.5-kHz bandwidth). Metabolite concentrations were determined by a method described previously
(28,
29). Briefly, the T
2 decay of the unsuppressed water signal from the PRESS experiment was measured at 10 different echo times to calculate metabolite concentrations corrected for the partial volume of CSF. This approach yields interindividual variations of about 10% and intrasubject variabilities of 3%–8% for the major metabolite peaks. The data were processed with a well-validated semiautomatic program
(28,
29). These measurements yield metabolite concentrations in “institutional units,” which were converted into millimolar concentrations with the use of published normal values
(29). For comparisons with the existing literature, we also determined metabolite ratios by using creatine as an internal standard.
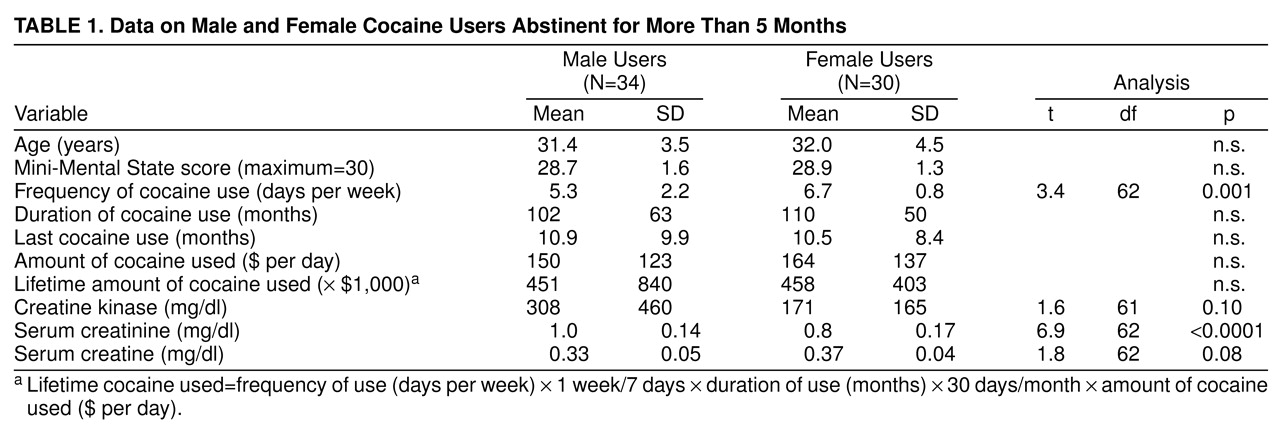
Statistical analyses were performed with Statview (Abacus, Inc., Berkeley, Calif.). Unpaired Student’s t tests were performed for the differences between male and female cocaine users on the variables shown in
table 1. Two-way analyses of variance (ANOVA) were performed to investigate the effect of the two independent variables, gender and cocaine use, on brain metabolite concentrations and metabolite ratios in both white matter and gray matter. Regression analyses of metabolite concentrations on lifetime cocaine use were performed with the use of a linear regression model. All values are reported as means and standard deviations; p values of 0.05 or less were considered significant.
RESULTS
All subjects had normal results on neurological examinations, negative HIV-1 serology, and negative urine toxicology screens. Five of the cocaine users (four female and one male) had a positive reactive protein reagent test for syphilis and received appropriate treatments; three male cocaine users were positive for sickle cell trait only on hemoglobin electrophoresis. Since these eight subjects showed no abnormalities on MRI and no significantly different cerebral metabolites in comparison with the other cocaine users, they were included in the final analyses. For the cocaine users, detailed histories of drug use revealed that 62% used marijuana intermittently, 74% used nicotine, and 72% used caffeine regularly; however, our exclusion criteria excluded subjects from this study if they were dependent on alcohol, amphetamines, or opiates.
All comparison subjects had normal MRI scans. However, several of the cocaine users showed minor abnormalities on MRI: two male cocaine users had small basal ganglia infarcts (globus pallidus and putamen), and three female cocaine users had small discrete periventricular white matter lesions, possibly due to microinfarcts. In addition, four female and four male cocaine users showed mild and diffuse white matter hyperintensities in the periventricular brain regions. Mild brain atrophy was observed in two male and three female cocaine users, primarily in the dorsal frontoparietal brain regions. Overall, there were no gender differences among the cocaine users with respect to MRI abnormalities.
Table 1 shows that the women used cocaine significantly more frequently than the men. The female users also had used cocaine for a longer period of time, had used larger amounts of cocaine per day, and consequently had higher total lifetime cocaine use than the male users; however, these differences were not significant. Both men and women had abstained from cocaine use for approximately 10 months before the study. The male users showed a significantly higher serum creatinine level (+20%), a nonsignificantly elevated creatine kinase level (+72.8%), and a nonsignificantly lower serum creatine level (–10.8%) than the female cocaine users.
In the frontal gray matter of all subjects, significant effects of cocaine on
N-acetyl compounds (–3.4%) and on myoinositol (+4.6%) were observed (
Table 2,
Figure 1). Gender effects were also observed, with the male subjects showing a higher level of choline-containing compounds (+4.5%), a higher percentage of CSF (+24%), a higher choline compound/creatine ratio (+5.6%), and a higher myoinositol/creatine ratio (+4.4%). However, there were no interaction effects between cocaine use and gender in the frontal cortex.
In frontal white matter, significant effects due to cocaine use were observed, with elevated myoinositol concentration (+10.8%) and myoinositol/creatine ratio (+11.7%) and decreased
N-acetyl compound/creatine ratio (–3.7%) (
table 2,
Figure 1). There were several significant interaction effects between cocaine use and gender: only male cocaine users showed an elevated creatine concentration (+5.7%) and a decreased
N-acetyl compound/creatine ratio (–6.6%) as a result of the increased creatine, while female cocaine users showed a more significant elevation of myoinositol/creatine ratio (+17.1%).
To avoid multiple correlations, total lifetime cocaine exposure was used for the correlation analyses. In all cocaine users, there was no significant relationship between brain metabolite concentration in either brain region with lifetime cocaine exposure. In the male users, cocaine use showed a significant effect on gray matter choline-containing compounds (r=0.34; F=4.08, df=1, 32, p=0.05). In the female users, although white matter myoinositol was elevated, no relationship with lifetime cocaine exposure was found (r=0.29; F=2.38, df=1, 32, p=0.10). Although frontal white matter creatine was elevated in the male users, there were no significant relationships with serum creatine, serum creatine kinase, or serum creatinine.
DISCUSSION
Crack cocaine abuse has been associated with a higher risk of cerebral infarcts
(3–
5). In this group of relatively young, abstinent, asymptomatic cocaine users, two male users showed “silent infarcts,” and three female users showed periventricular white matter lesions, which may be related to cocaine abuse. In addition, mild brain atrophy was observed in two male and three female cocaine users, which may be a normal variant or an early sign of alcoholic atrophy, although these subjects denied a history of alcohol dependence. Since only a few subjects showed structural abnormalities, a gender-specific effect of cocaine on the brain cannot be concluded from MRI alone. Furthermore, the percentage of CSF within the gray matter voxels, which may yield an estimate of brain atrophy, showed only an effect of gender and not an effect of cocaine. Therefore, structural imaging is less sensitive than
1H-MRS, which shows significant effects of cocaine on neurochemicals.
This study found persistent
1H-MRS abnormalities in the frontal lobes of abstinent cocaine-dependent subjects.
N-acetyl compounds were reduced in the frontal gray matter, a brain region where functional deficits in cocaine users have previously been described
(8,
12,
30). Since
N-acetylaspartate, constituting 80% of the
N-acetyl compound peak, is considered a neuronal marker
(21), this finding indicates neuronal damage in the frontal cortex due to crack cocaine use. Although it is possible that there are other causes of neuronal injury in this subject population, our study criteria screened out subjects with excess alcohol use, a history of dependence on other drugs, and other neuropsychiatric illnesses.
Both the male and the female cocaine users also had increased concentrations of myoinositol in their frontal lobes. This elevated myoinositol level most likely represents glial hypertrophy or proliferation, since myoinositol has been shown to be present only in glial and not neuronal tissue
(23). Glial cells are involved in protective and restorative function; therefore, evidence for increased glial activity (with increased myoinositol) implies a reactive process in the brains of cocaine users. Elevated myoinositol levels have also been observed in numerous other brain disorders, such as Alzheimer’s disease
(31,
32), multiple sclerosis
(33), HIV-1-associated dementia
(34,
35), progressive multifocal leukoencephalopathy
(36), myotonic dystrophy
(37), and effects of 3,4-methylene dioxymethamphetamine (MDMA) use
(38). All of these diseases or conditions are associated with either gliosis or cell membrane injury. Comparable to early stages of Alzheimer’s or HIV-associated dementia, the elevated myoinositol in our cocaine users was mild in the cortex (+4.6%) but moderate in the white matter (+10.8%).
Because glial cells have higher creatine levels than neuronal cells
(23), glial hypertrophy might also explain the increased creatine in the white matter of male cocaine users. Alternatively, the increased creatine levels might be caused by increased cerebral uptake of creatine, since creatine is synthesized only in the liver. To investigate this possibility further, we measured serum creatine, creatinine, and creatine kinase. However, we did not find abnormal levels of serum creatine or creatinine, or significant correlations with brain creatine, in our subjects.
The MRS findings in our male cocaine users are similar to the previous
1H-MRS findings in a group of male African American cocaine users
(7). As in the previous study, we found significantly increased myoinositol in both the gray and white matter of the male cocaine users. The findings differ slightly with respect to creatine: the previous study showed a 7% increase in creatine in both temporoparietal white matter and occipital gray matter, whereas the current study showed increased creatine (+5.7%) only in the frontal white matter and not in the gray matter of the male cocaine users. In contrast to the previous study, the current male cocaine users also showed decreased
N-acetyl compounds (–4.8%) in the frontal gray matter.
The differences between the findings of the two studies might be due to several factors. First, different brain regions were examined (frontal lobe versus occipital and parietal lobe). Therefore, the decreased
N-acetyl compounds observed in the frontal lobe of the cocaine users may not be present in the other brain regions. This finding is consistent with previous reports of decreased dopamine receptors with associated hypometabolism in the frontal lobes of cocaine users
(14,
30). Second, our current study group is younger than the one studied previously. The difference in myoinositol abnormalities (+8.3% in the frontal white matter of the current younger male users compared with +18.1% in the parietal white matter of the older male cocaine users) might therefore be caused either by a regional effect or by more extensive effects of cocaine in the older subjects (including subjects between 40 and 50 years of age). Finally, some of the differences in creatine concentration between our first and second studies might be related to differences in the neuronal damage observed. Creatine is present in neuronal as well as in glial tissue; therefore, neuronal loss might cause some decreases in creatine. In fact, we did not observe increased creatine in the frontal gray matter of the male cocaine users (the region with significantly reduced
N-acetyl compounds), whereas there was an increase in creatine in the white matter.
The MRS abnormalities in the male cocaine users were different from those observed in the female cocaine users. In gray matter, although the decreased
N-acetyl compounds and elevated myoinositol were more pronounced in the male users (–4.8% for
N-acetyl compounds and +5.3% for myoinositol) than in the female users (–2.0% for
N-acetyl compounds and +3.8% for myoinositol), no significant interaction due to gender was observed. Decreased
N-acetyl compounds suggest neuronal injury, while elevated myoinositol suggests glial activation, possibly associated with reactive or repair processes. Although our findings did not reach statistical significance, they parallel those of Levin et al.
(8), who found that cocaine-dependent women had fewer perfusion abnormalities in the frontal lobe than cocaine-dependent men. The etiology of this gender effect of cocaine is unknown; conditions associated with female gender (e.g., hormonal differences) may have a protective effect for cerebral insult due to cocaine. Furthermore, since brain injury due to cocaine may be responsible for relapses, the gender differences in brain metabolite effects may explain and support previous studies which showed that female cocaine users had better treatment outcome and were more able to remain abstinent in follow-up than male users
(9,
10). Future studies with a larger study group are needed to determine whether such an interaction effect exists between the effects of gender and cocaine use.
In frontal white matter, only male cocaine users showed a significantly decreased
N-acetyl compound/creatine ratio (–6.6%), due to minimally decreased
N-acetyl compounds (–2.6%) and elevated creatine (+5.7%). In contrast, only the female cocaine users showed a significant effect of elevated myoinositol/creatine ratio (+17.1% in the female cocaine users, compared with 6.8% in the male users) and a nonsignificant but higher elevation of myoinositol in frontal white matter (+13.5% in the female users and +8.3% in the male users). As discussed above, the elevated creatine in the male users confirms our previous observations in another male cocaine-dependent population
(7). The higher elevation of myoinositol in the cocaine-dependent women suggests a stronger glial reactive process in their brains compared with those of the men. These gender-specific changes in neurochemicals were present despite a nonsignificant difference between the two groups in lifetime cocaine use.
In summary, we confirmed our previous observation that male cocaine users have elevated levels of creatine and myoinositol in white matter. In addition, we found decreased N-acetyl compounds, indicative of neuronal loss or dysfunction, and elevated myoinositol in the frontal gray matter of cocaine users. In the female users, however, the only 1H-MRS abnormality observed was elevated myoinositol in frontal white matter. Our findings show that the brains of cocaine-dependent men and of cocaine-dependent women respond differently to the effects of cocaine. Therefore, gender and regional differences in brain metabolite concentration are important variables to consider in future 1H-MRS studies of drug abuse. Since quantitative 1H-MRS can evaluate the extent of brain injury associated with cocaine abuse, it may have a role in the prognosis or monitoring of treatment effects.