Children of alcoholics are at higher risk for alcoholism
(1); therefore, they have been the focus of studies on subjective, psychomotor, physiological, and biochemical responses to ethanol
(2,
3). Previous studies of less sensitivity to benzodiazepine and ethanol in children of alcoholics than in comparison subjects have yielded conflicting results
(4–
7). The genetic and neurobiological mechanisms of diminished sensitivity to benzodiazepine and ethanol in children of alcoholics are unclear, probably because these drugs affect multiple neurotransmitter systems
(8).
Recently, we detected a novel Pro385Ser amino acid substitution polymorphism of the GABA
Aα6 subunit gene (9). Frequency of the more rare Ser385 allele was approximately 0.04 in Finnish Caucasians. Although the substitution is nonconservative, no information was available on function. Cross-tolerance of benzodiazepine with ethanol and the efficacy of benzodiazepines in treating alcohol withdrawal suggest that GABA
A receptors have a key role in alcohol’s effects. In a selective genotyping study
(10), we found that the GABA
Aα6 genotype correlated with level of response to alcohol. Our aim in the current study was to evaluate the hypothesis that Pro385Ser is associated with differences in benzodiazepine sensitivity.
METHOD
We studied 51 unrelated Caucasian offspring of alcoholic fathers; 25 were sons, and 26 were daughters. Their mean age was 22.2 years (SD=2.0, range=18–25). Psychiatric disorders were diagnosed by using the Structured Clinical Interview for DSM-III-R. The proband and at least one first-degree relative were interviewed with the Family Informant Schedule and Criteria (an extended version of the Family History Research Diagnostic Criteria) and with the Family History Assessment Module from the SSAGA (a structured diagnostic interview developed in the Collaborative Study on the Genetics of Alcoholism). Children of alcoholics were free of lifetime DSM-III-R axis I or axis II psychiatric or substance use disorders, except adjustment disorder.
The subjects were medically healthy and reported that they had taken no medication for at least 1 month and that they had not used a benzodiazepine more than once. All subjects gave written informed consent before participating in the study after all procedures and risks were explained. The study was approved by the University of Washington Institutional Review Board.
The subjects were tested on 2 days about 1 week apart. Intravenous catheters were inserted in an antecubital vein in each arm. One intravenous line was used for blood sampling for diazepam levels, and the contralateral arm was used for administration of diazepam or placebo. Infusions were given double-blind and in randomized order. On the diazepam day, the drug was administered at 15-minute intervals in four doses of 25, 25, 50, and 100 µg/kg, yielding logarithmically increasing cumulative doses of 25, 50, 100, and 200 µg/kg. On the placebo day, saline was injected in similar volumes and at the same times.
On each testing day, both eye movements and diazepam levels were measured at each of 12 time points: twice at baseline, once after each of the four doses, and six times at 30-minute intervals after the last dose. Peak saccadic eye movement velocity and average smooth pursuit eye movement gain were recorded by using a noninvasive infrared oculographic device (Eye Trac model 210, ASL Laboratories, Waltham, Mass.).
A 365-bp genomic DNA fragment was amplified with primers 5′-CTGACTCCAAATATCATCTG-3′ and 5′-GAGAAGCATCTACACAAGTC-3′ (primer annealing at 60°C)
(9) and digested with Fok I, yielding two fragments 261 nt and 104 nt in size in the case of Ser385, as described elsewhere
(9).
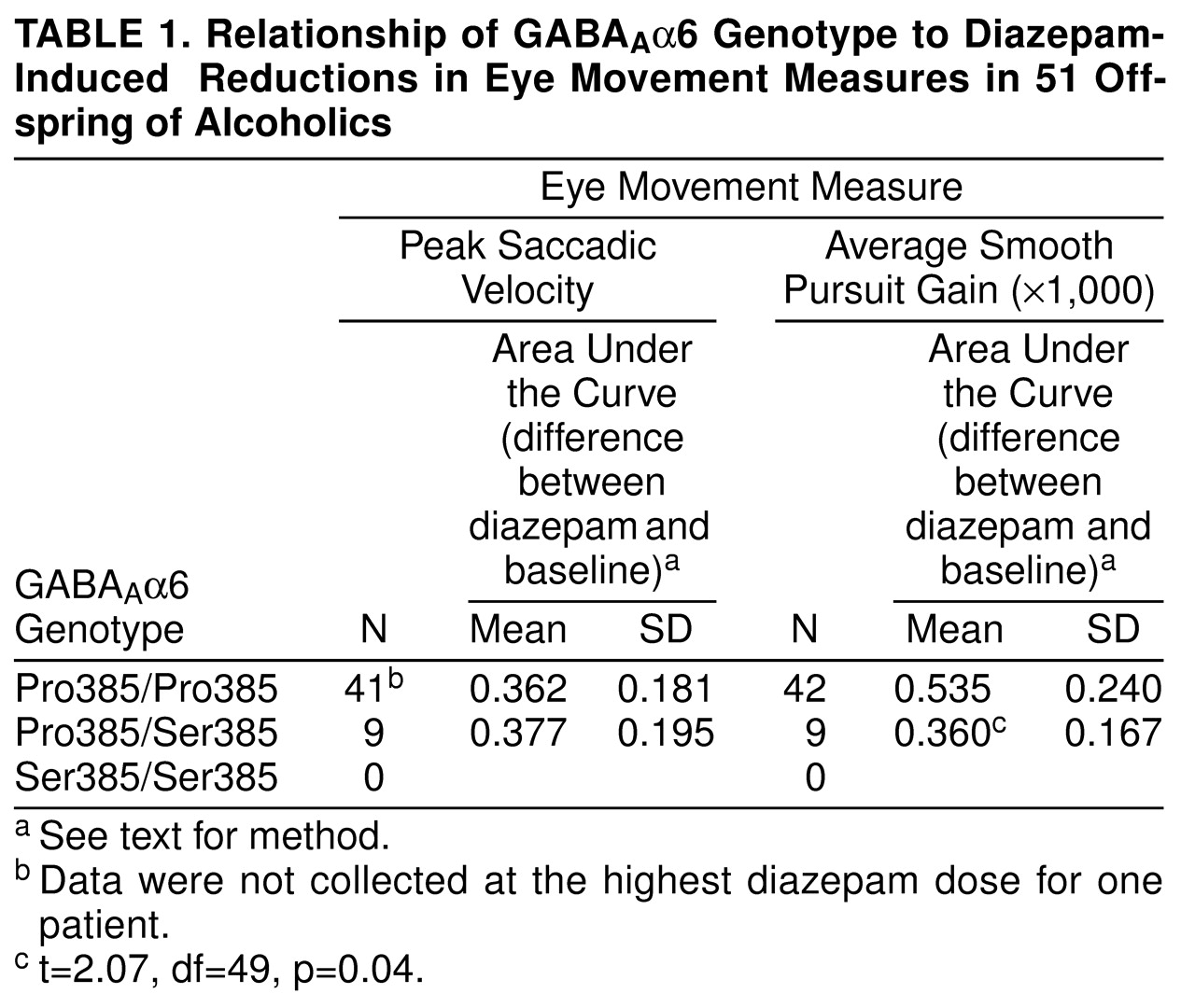
Sensitivity of saccadic eye movement velocity and smooth pursuit gain was measured both at baseline and after each of four diazepam doses. The baseline-dose differences were used to construct, by a trapezoidal technique, the area under the curve. The area under the curve for the dependent variable was then divided by the area under the curve for plasma diazepam levels to correct for individual variability in diazepam levels.
These area-under-the-curve ratios were compared between children of alcoholics with the Pro385/Pro385 homozygous genotype and the Pro385/Ser385 heterozygous genotype by using t tests. There were no Ser385/Ser385 homozygotes.
DISCUSSION
Among the 13 subunits known to participate in the formation of GABA
A/benzodiazepine receptor complexes in mammals
(11), the α6 subunit is unique in its benzodiazepine-agonist-insensitive pharmacology and in its restricted distribution
(12). GABA
Aα6 expression is limited to cerebellar granule cells. An enhanced potentiation of GABA
A currents by diazepam binding to the receptor complex containing the Pro385 variant could underlie the differences in average smooth pursuit eye movement gain seen in subjects of different genotypes. The observation by Korpi et al.
(13) that a rat GABA
Aα6 variant is involved in differential alcohol sensitivity and diazepam response of the receptor helped generate the hypothesis that a structural variant might be present in the human GABA
Aα6 that could affect sensitivity to these drugs. In a selective genotyping study
(10), we have also found that level of response to alcohol in humans correlates with GABA
Aα6 genotype. Again, Pro385/Ser385 heterozygotes were less sensitive than Pro385/Pro385 homozygotes. Despite these converging lines of evidence supporting support a role for genetic variation in GABA
Aα6 in alcoholism and diazepam sensitivity, alteration of GABA
Aα6 function by the human Pro385Ser variant has not been demonstrated. However, this is the first indication that a difference in human diazepam response may arise from a naturally occurring variant in a GABA
A receptor subunit.
We did not find any association between Pro385Ser and saccadic eye movement. Saccadic eye movements are mediated by the parietal cortex and the frontal cortex and through associated connections with the basal ganglia as well as the superior colliculus and, finally, premotor areas in the pons. Meanwhile, and consistent with the narrow localization of GABA
Aα6 to the cerebellum, the smooth pursuit eye movements that were associated with the Pro385Ser genotype are predominantly mediated by the cerebellum, as well as other cortical areas
(14). Therefore, the association of Pro385Ser to pursuit eye movements but not to saccadic movements corresponds to the restricted expression of GABA
Aα6 to the cerebellum.