Dysfunctional serotonergic systems have been associated with aggressive behaviors in animal
(1) and human adult studies
(2) . However, results of studies on the role of serotonin in childhood aggression have been inconclusive
(3,
4) .
The serotonin transporter gene (5-HTT or SLC 6A4) mediates the magnitude and duration of serotonin (5-HT) synaptic signaling
(5) . Polymorphisms lead to changes in protein function and/or expression
(6) . Thus, the serotonin transporter promoter polymorphism (5-HTTLPR) and 5-HTT variable-number-tandem-repeat polymorphisms, and their products, may serve to modulate aggressive behavior.
5-HTTLPR is a 44-base pair insertion/deletion recently shown to be triallelic, due to the discovery of the Lg allele that behaves codominantly relative to the La and S variants
(7,
8) . It should, however, be noted that in an earlier study, Nakamura et al.
(9) reported 10 allelic variants for 5-HTTLPR. Lg results from a single-base substitution (A>G) and possesses a binding site for a transcription factor that appears to suppress 5-HTT transcription
(7,
8) . Lg is “low transcribing” (similar to S). Further evidence of the functional activity of the Lg polymorphism can be found in a recent report
(10), demonstrating that this polymorphism (RS 25 531) altered binding of nuclear extracts to a consensus sequence for the activator protein 2 transcription factor, which is believed to be a critical factor in regulating neural gene expression in mammals.
5-HTT variable-number-tandem-repeat consists of three alleles composed of nine, 10, or 12 copies of the 17-base pair repeat, with the nine allele being rare
(11) . The polymorphism may regulate the transcription of the 5-HTT gene since the 12-repeat allele displays stronger enhancer capabilities
(12) compared to the 10-repeat allele.
When the 5-HTT variable-number-tandem-repeat and the 5-HTTLPR genotypes were grouped as “high expressing” or “low expressing,” a combined effect on 5-HTT gene expression was observed
(5) .
Method
Clinically referred children ages 5–15 (70 boys and 12 girls: mean age, 9.54 [SD=2.62] years) displaying aggressive behavior for at least 2 years participated in the study. Inclusion criteria consisted of scores at or exceeding the 90th percentile on the aggressive subscales of both the Child Behavior Checklist
(13) and the Teacher’s Report Form
(14) . Exclusion criteria included chronic medical illness, neurological disorder or schizophrenia, mania, autism or other pervasive developmental disorders, Tourette’s disorder, and IQ less than 80.
DSM-IV diagnoses for disruptive behavior disorders were obtained through administration of the National Institute of Mental Health Diagnostic Interview Schedule for Children
(15) to the participant’s primary caretaker and reviews of the participants’ health records. Of the 98% of the sample for whom diagnostic information was available, all qualified for at least one disruptive behavior disorder and 74% for attention deficit hyperactive disorder. There were no participants who displayed adolescent-onset disruptive behavior disorders; all were first apparent during childhood. Based on the Children’s Depression Inventory, less than 5% of the subjects obtained clinically elevated depression scores (i.e., total t score > 65).
Participants donated venous blood or cheek swabs as deoxyribonucleic acid (DNA) samples. DNA extraction from whole blood was completed following the nonenzymatic, high-salt procedure
(16), while extraction from buccal epithelial cells was performed using the Qiagen DNA isolation kit. The Centre for Addiction and Mental Health Research Ethics Review Board approved this study, and written informed consent was obtained from all participants.
Participants and gender- and ethnicity-matched adults (free of major psychopathology) were genotyped for the 5-HTTLPR (N=77) and 5-HTT variable-number-tandem-repeat (N=78) polymorphisms. While there were a total of 82 participants, not all could be genotyped for both markers. Of the 82, 77 could be genotyped for 5-HTTLPR, and 78 could be genotyped for 5-HTT variable-number-tandem-repeat. Polymerase chain reaction primers and conditions followed those previously described
(17) . To identify Lg, the polymerase chain reaction product was digested with 10 U of Msp I (New England Biolabs) overnight at 37°C, resolved on a 3.5% agarose gel (containing ethidium bromide), and visualized at the 1-hour and 1.5-hour mark with ultraviolet light. It should be noted that the allele frequencies in our comparison group were within the range of the population allele frequencies reported by Hu et al.
(7) .
Two-tailed chi-square analyses were performed to assess genotype frequencies among aggressive children and healthy comparison subjects and to determine whether combined 5-HTTLPR and 5-HTT variable-number-tandem-repeat genotypes are related to childhood aggression. All reported p values have been corrected for multiple testing of the two serotonin transporter markers (5-HTTLPR and 5-HTT variable-number-tandem-repeat) using Bonferroni correction
(18) . Confidence intervals (CI) were set at 95%. Genotypes were grouped according to their hypothesized level of activity: “high expressing” (12/12 and La/La), “intermediate” (La/S, La/Lg) and “low expressing” (10/10, 10/12, S/S, Lg/S, Lg/Lg). The 5-HTTLPR and 5-HTT variable-number-tandem-repeat genotypes were classified independently, with a second laboratory technician blindly reviewing genotypic groupings. The nine allele of the 5-HTT variable-number-tandem-repeat was excluded from all genotype analysis due to its low frequency and its unknown level of functional activity, reducing the sample size for this polymorphism to 73.
Results
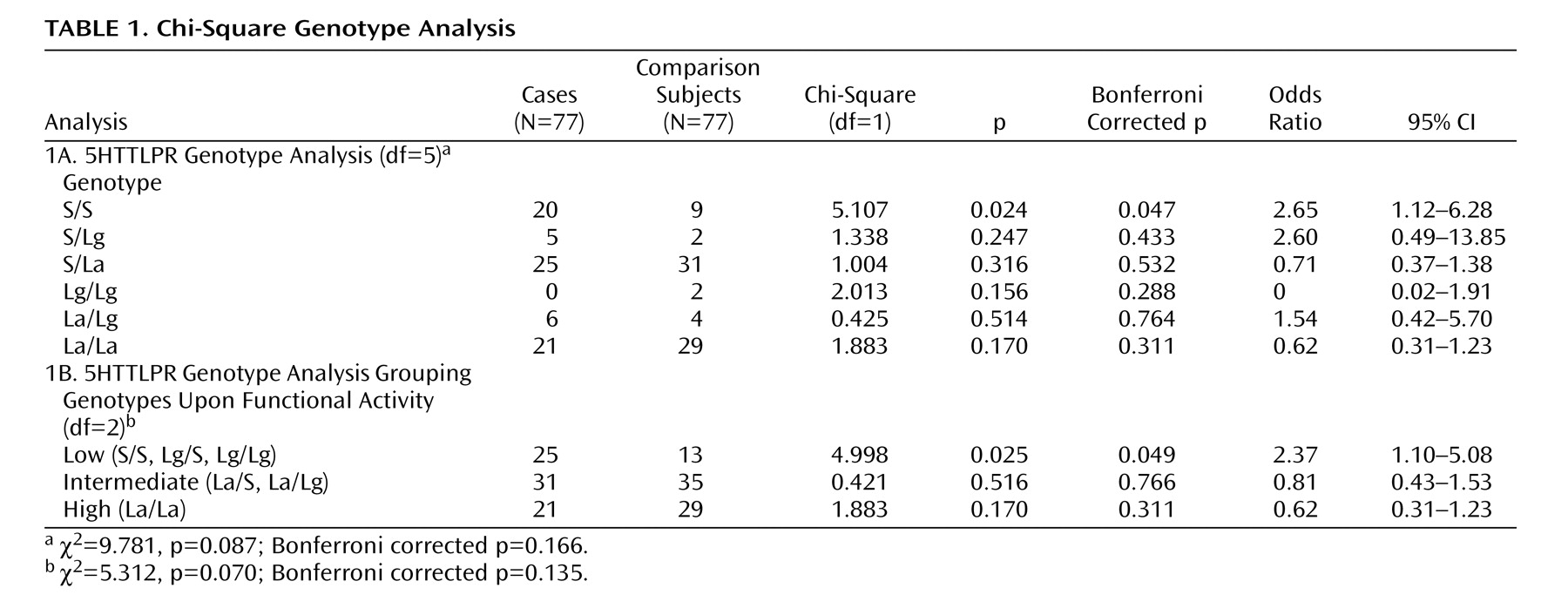
A significant positive association was found between aggressive behavior and the “low expressing” genotypes (S/S, Lg/S, Lg/Lg) (N=77, p=0.049, OR=2.37, CI=1.10–5.08) as well as with the S/S genotype alone (p=0.047, OR=2.65, CI=1.12–6.28) (
Table 1 ). The Lg allele count was 11 in the participants and 10 in the comparison subjects; thus, it did not have a major impact on the association. No association was found for the “intermediate” or “high expressing” 5-HTTLPR genotypes or when the 5-HTTLPR and 5-HTT variable-number-tandem-repeat polymorphisms were combined according to their functional activity. Similarly, the genotypes of 5-HTT variable-number-tandem-repeat polymorphisms were not found to be significantly associated with childhood aggression (N=73, p=0.730). For participants and comparison subjects, respectively, the frequencies for the 10/10 genotype were 14 and 13; for the 10/12 genotype, 26 and 33; and for the 12/12 genotype, 33 and 27.
Discussion
This is the first study to demonstrate a significant association between the 5-HTTLPR gene and aggression in children. The “low expressing” genotype group (S/S, Lg/S, Lg/Lg) is significantly more prevalent in aggressive children and is associated with more than twice the risk of aggressive behavior. These results remained significant even after correcting for multiple testing of the two markers (5-HTTLPR and 5-HTT variable-number-tandem-repeat), a conservative correction considering that the two markers are not fully independent. This finding is consistent with models of aggression, which associate decreased central serotonin activity with aggressive behaviors
(3) . It should, however, be noted that an increase or decrease in transporter function is not necessarily associated with a corresponding increase or decrease in central serotonin function.
Caspi et al.
(19) found an association between the S allele of the 5-HTT promoter and depression in adults in relation to stressful life events. While very few of our cases displayed symptoms of depression, it is possible that the S allele may confer a general vulnerability/diathesis that may manifest itself as either depression or aggression. It is also possible that there is a developmental gradient such that in childhood, aggression is prominent in this sample selected for high aggression, but as adults, depressive symptomatology may become more prominent. This is an area that clearly warrants further research.
Our selection of children with extreme, persistent, and pervasive aggression, together with the more complete genotyping of the 5-HTTLPR gene, resulted in a significant relation between childhood aggression and the “low expressing” genotypes of the serotonin transporter polymorphisms. Had we not genotyped for the Lg allele, which had previously been included as an La allele, its “low transcribing” activity would be expected to have moderated the difference between the S and La alleles.
A limitation of this study is that comparison subjects were adults reporting retrospectively on their mental health during childhood. While there is considerable literature indicating that children with a history of extreme, pervasive aggression have a high likelihood of exhibiting antisocial behavior as adults
(20,
21), it is possible that some of our comparison subjects may have been aggressive as children. It is, however, unlikely many would have met the stringent criteria that were required for the child participants in this study. Most importantly, if some of the comparison subjects had been aggressive as children, it would have been more difficult to demonstrate a difference between cases and comparison subjects, making our results more conservative.
In addition, the possibility of population stratification exists, despite matching comparison subjects on gender and ethnicity. Given the relatively small sample, these results should be considered preliminary, and replication of these findings is necessary.